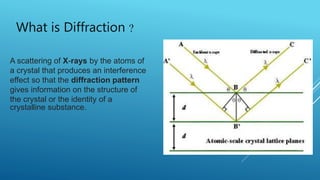
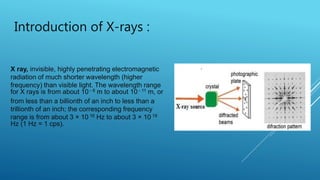
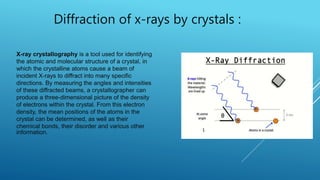
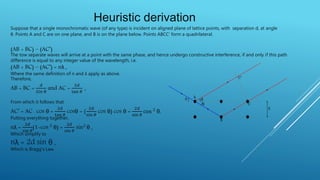
This document discusses the diffraction of X-rays by crystals. It begins by defining diffraction as the scattering of X-rays by crystal atoms that produces an interference pattern, giving information about the crystal structure. It then introduces X-rays as highly penetrating electromagnetic radiation with wavelengths between 10-8 to 10-11 meters. The document notes that X-ray crystallography was initiated in 1914 by W.H. Bragg and W.L. Bragg to study atomic crystal structures using X-ray diffraction. It explains that X-rays diffract into specific directions when incident on a crystal due to the crystalline atoms, allowing determination of electron density and atomic positions. Bragg's Law relating diffraction angle and interplanar spacing is then heur