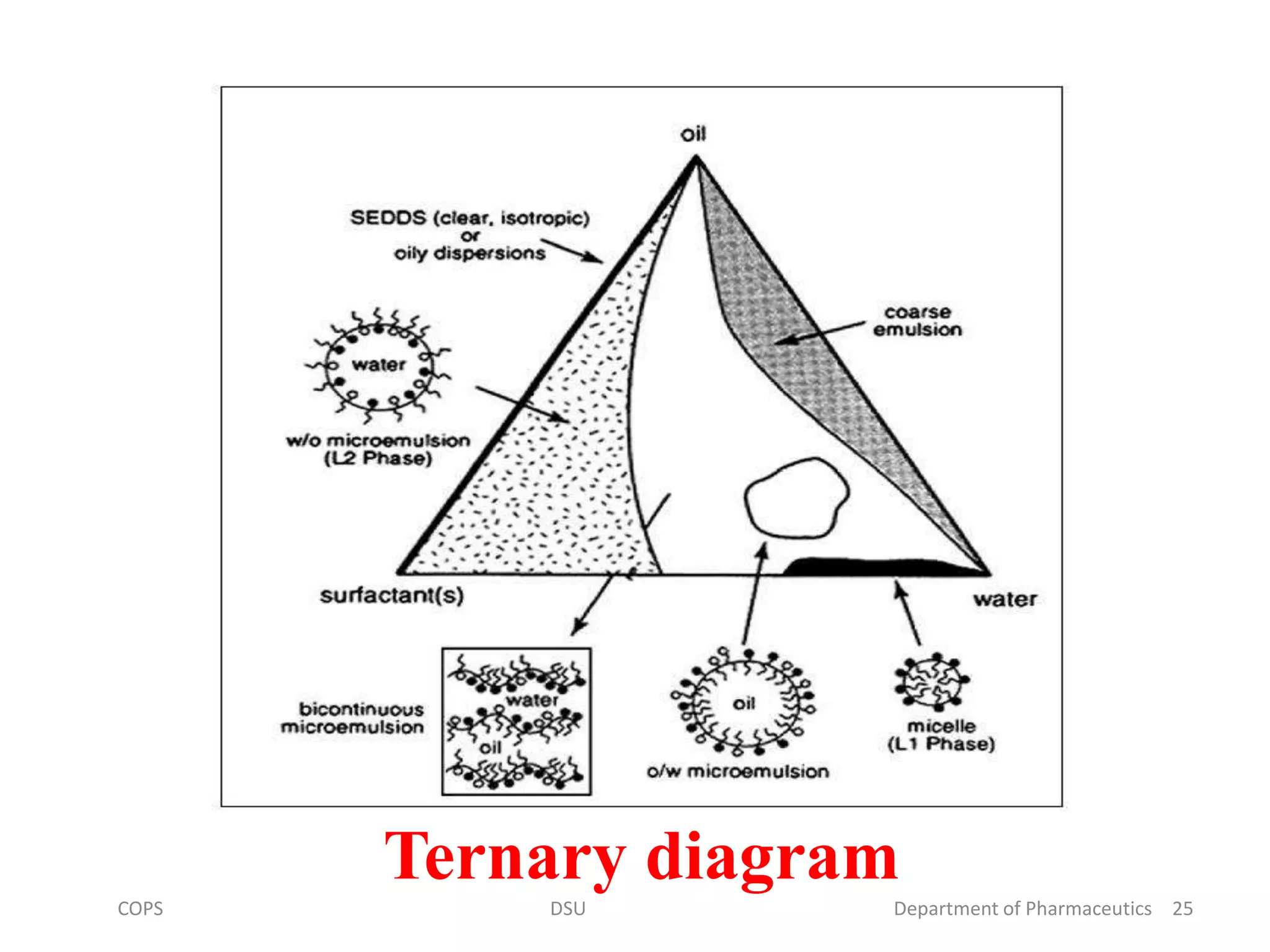
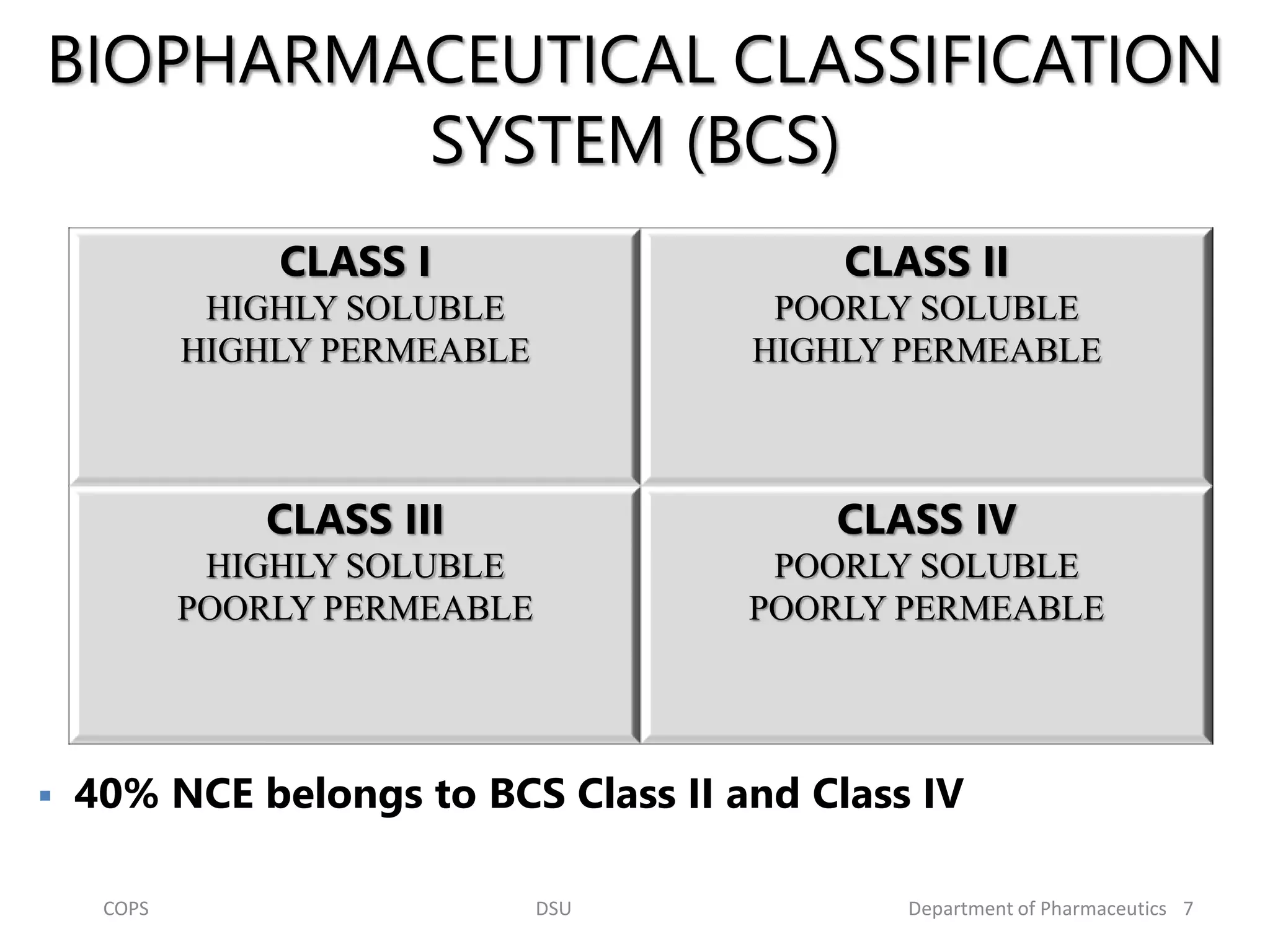
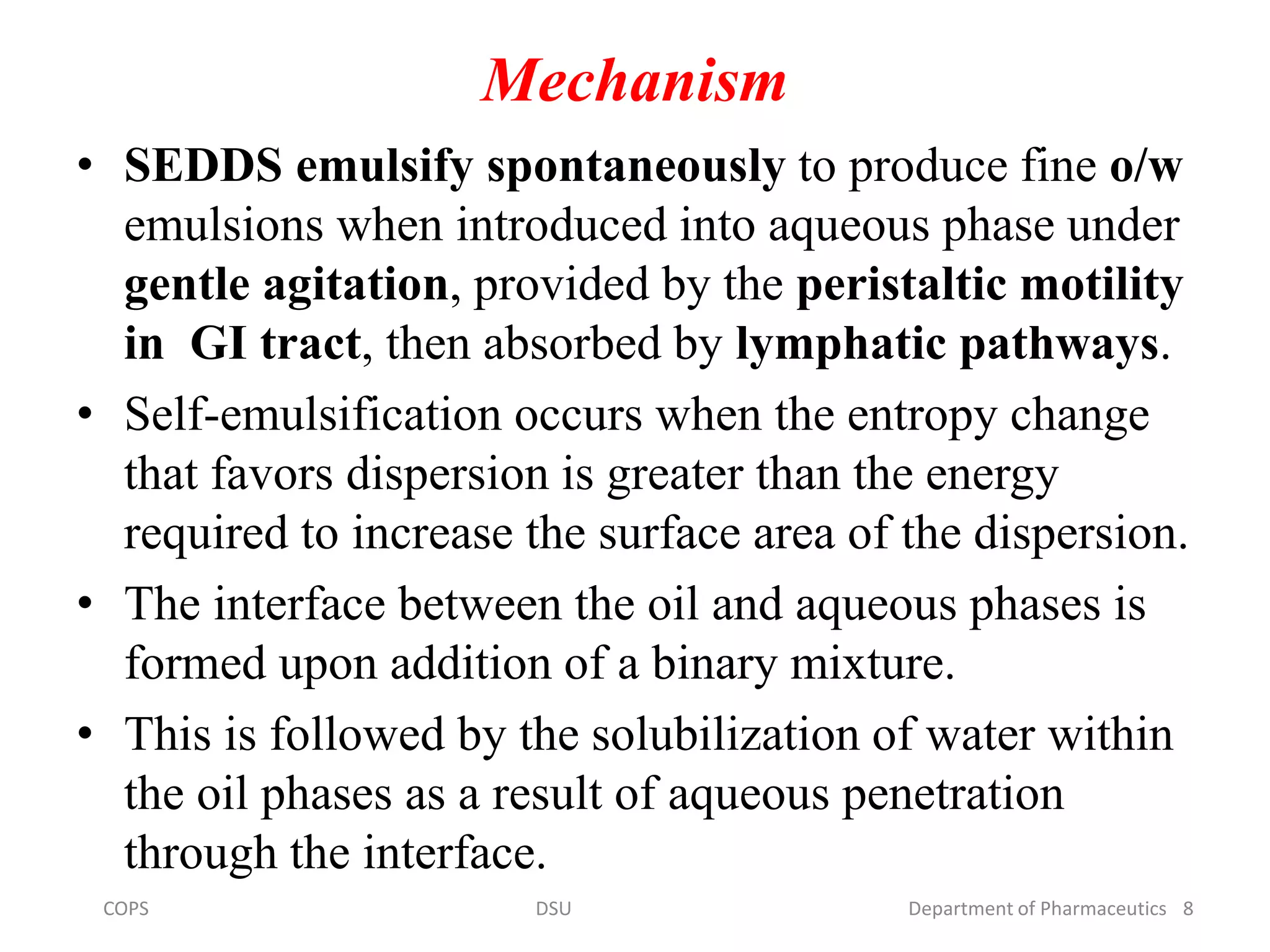
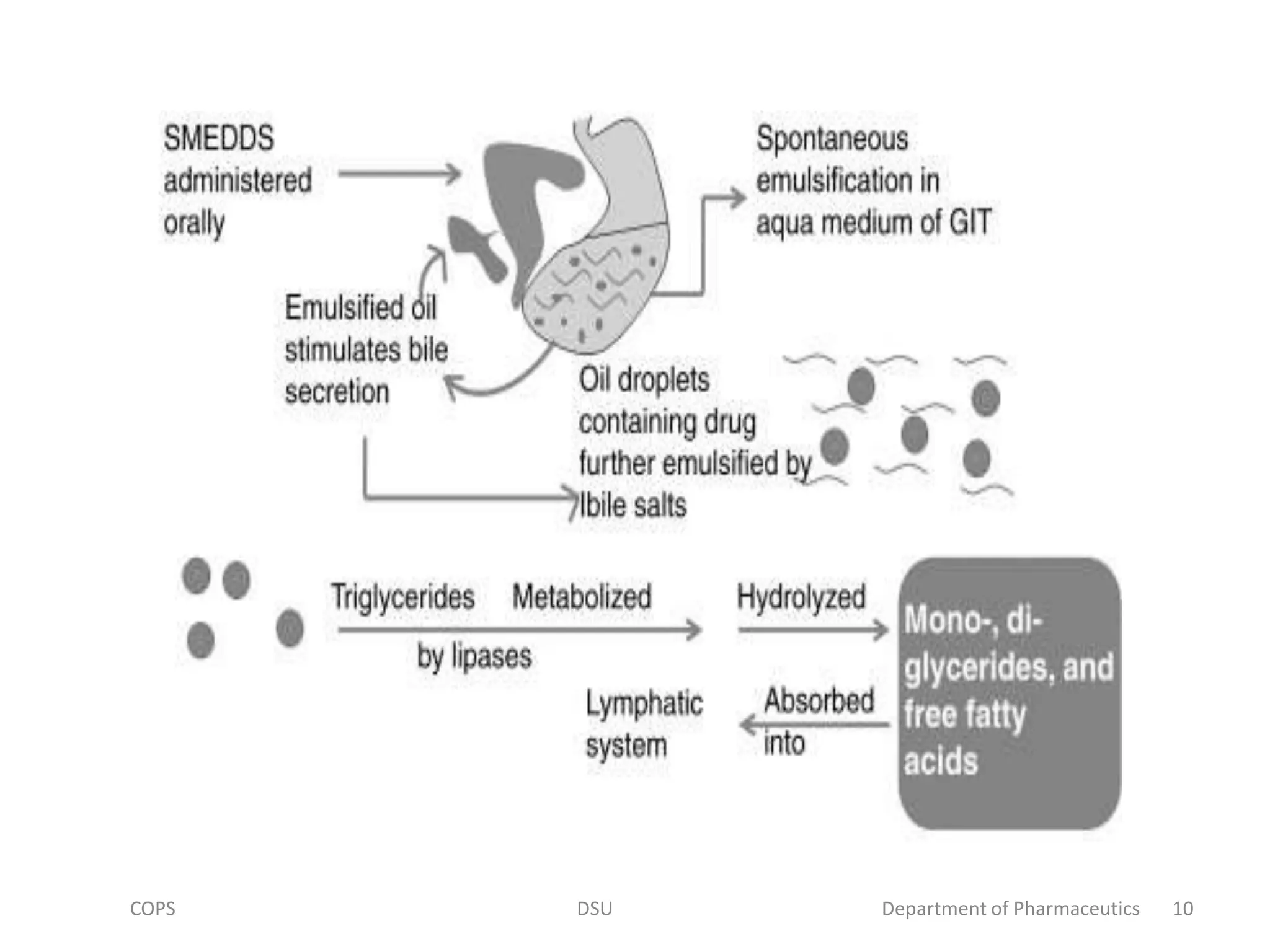
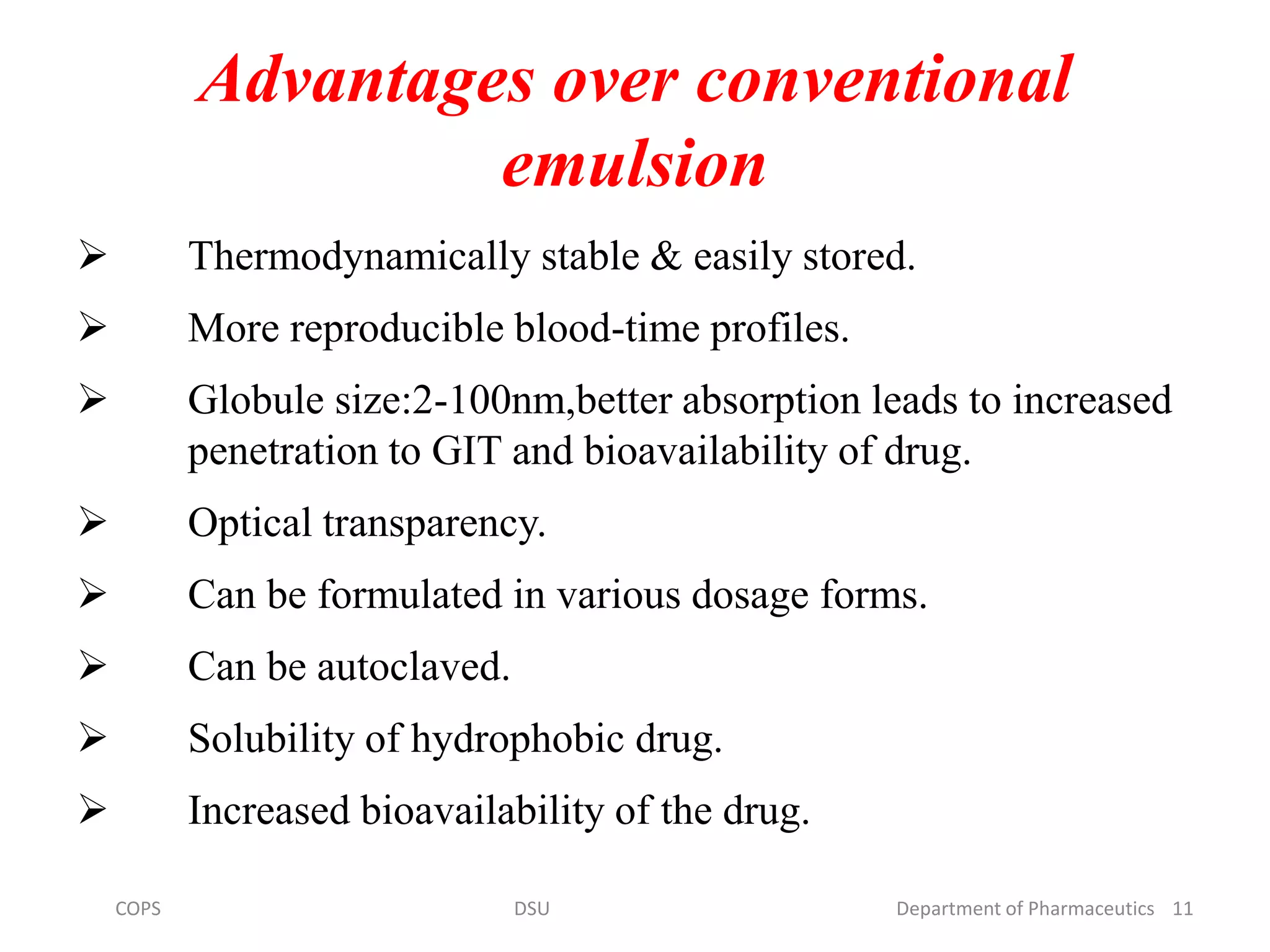
The document discusses self micro-emulsifying drug delivery systems (SMEDDS), highlighting their composition, advantages, and disadvantages in enhancing the oral bioavailability of poorly soluble drugs. It compares SMEDDS with other formulations like self-emulsifying drug delivery systems (SEDDS) and conventional emulsions, emphasizing their thermodynamic stability and smaller droplet size. Various formulation techniques, screening of excipients, and applications in drug delivery are also addressed.









![Other components
• pH adjusters
• Flavours
• Antioxidant agents
– Lipophilic antioxidants(E.g. alpha tocopherol,
Propyl Gallate,Ascorbyl palmitate )
– [ stabilize the oily content of SMEDDS
formulation].
• Consistency builder
21COPS DSU Department of Pharmaceutics](https://image.slidesharecdn.com/smedds-191119134402/75/self-micro-emulsifying-drug-delivery-system-21-2048.jpg)