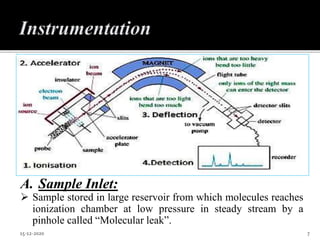
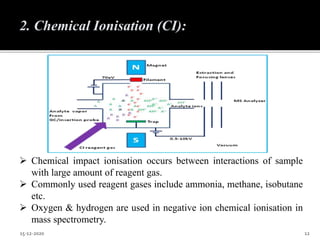
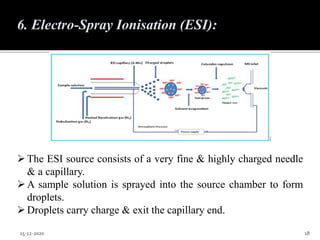
The document provides an overview of mass spectroscopy, an analytical technique for studying ionized molecules to determine molecular weight, structural characterization, and qualitative and quantitative analysis. It details the basic principles, working mechanisms, and various ionization techniques and mass spectrometric analyzers used in the process. Applications of mass spectroscopy across different fields such as environmental monitoring, geochemistry, and drug analysis are also discussed.