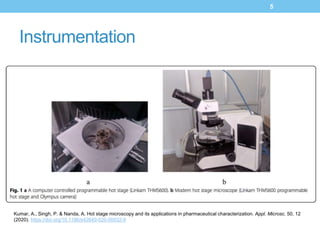
The document provides an overview of hot-stage microscopy (HSM), which couples thermal analysis with optical microscopy to observe solid-state materials as a function of temperature and time. HSM is used to support DSC and TGA and detect small changes missed by other techniques, such as desolvation and recrystallization. The instrumentation consists of a computer-controlled hot stage, optical microscope, and camera. HSM has various applications in pharmaceuticals for morphology studies, polymorphism, cocrystal screening, and detecting incompatibilities. It allows visual observation of processes like solid-solid transitions, phase changes, and desolvation.