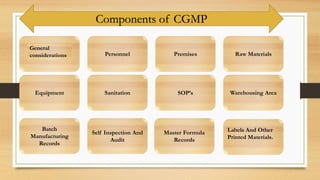
The document discusses Current Good Manufacturing Practices (CGMP) regulations enforced by the FDA to ensure drug products' identity, strength, quality, and purity. It outlines the history, objectives, requirements for facilities, personnel, equipment, and packaging, as well as the FDA's recall system to protect public health against defective products. The content also covers the classification of over-the-counter (OTC) drugs, safety regulations, and tamper-resistant packaging guidelines.