- Generic drugs are comparable to brand name drugs in dosage, quality, and intended use but are cheaper. They contain the same active ingredients as original formulations.
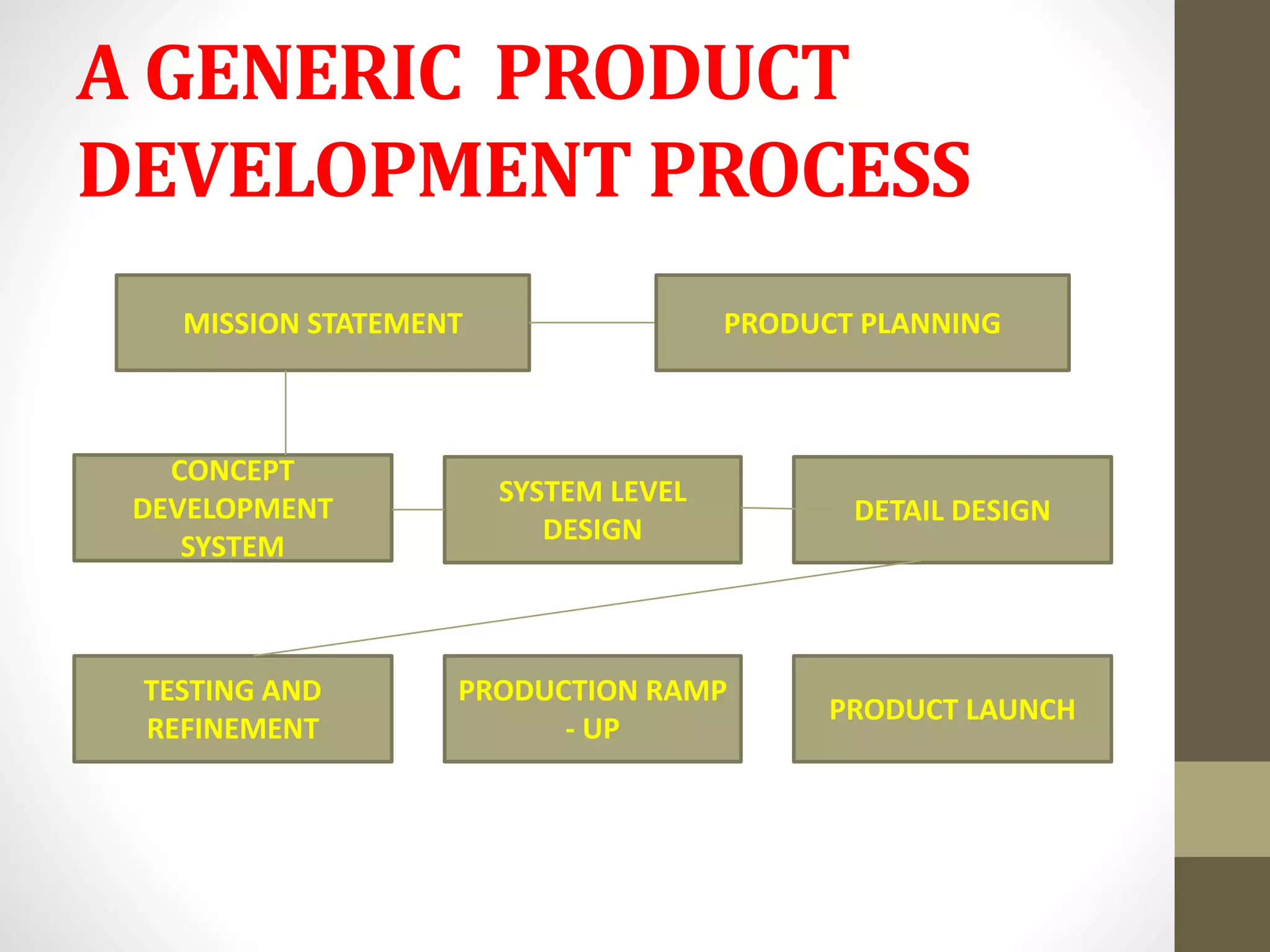
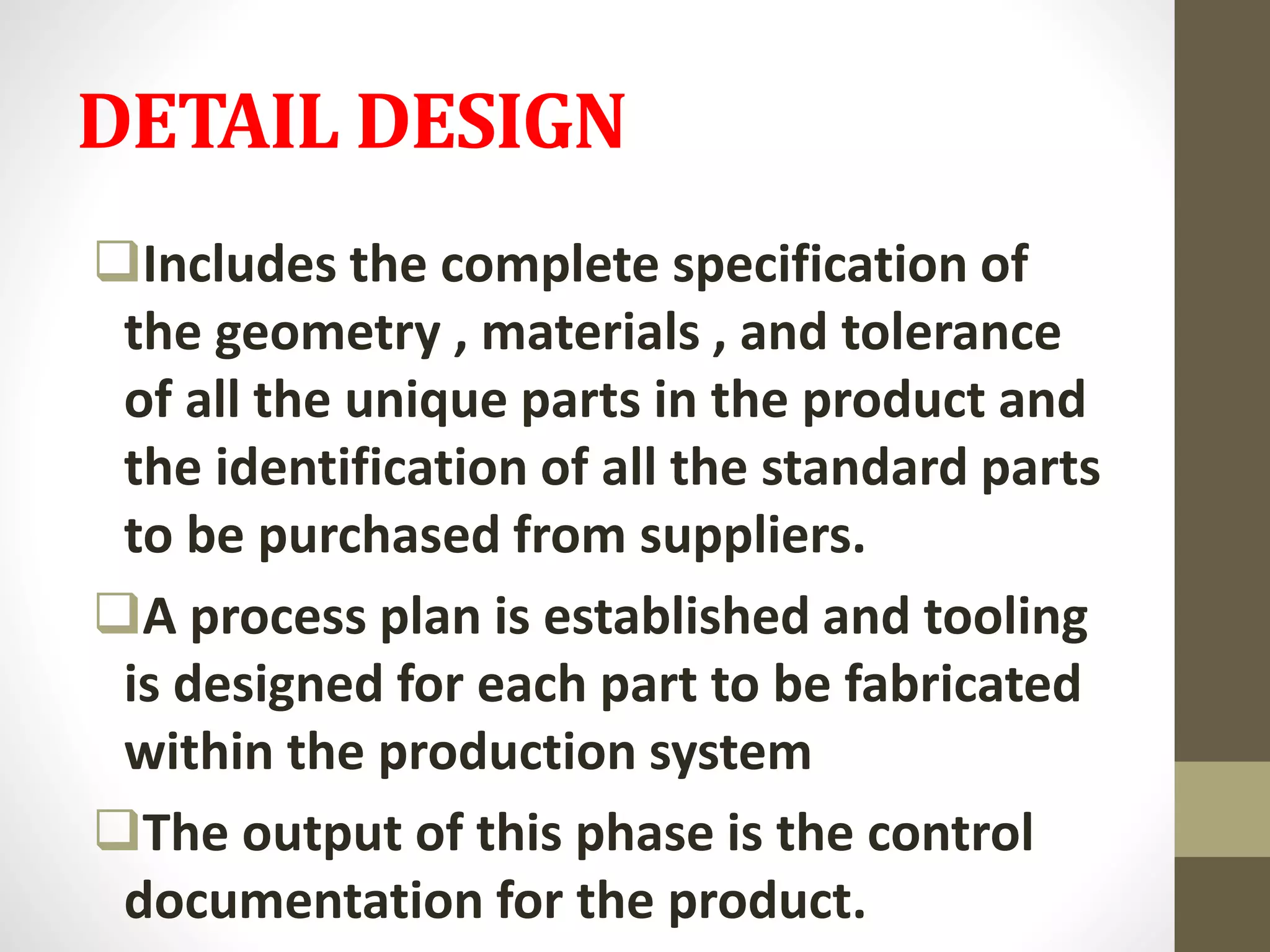
- The generic drug development process involves concept development, system-level design, detail design, testing and refinement, production ramp-up, and product launch. It aims to develop affordable drugs that balance public health needs.
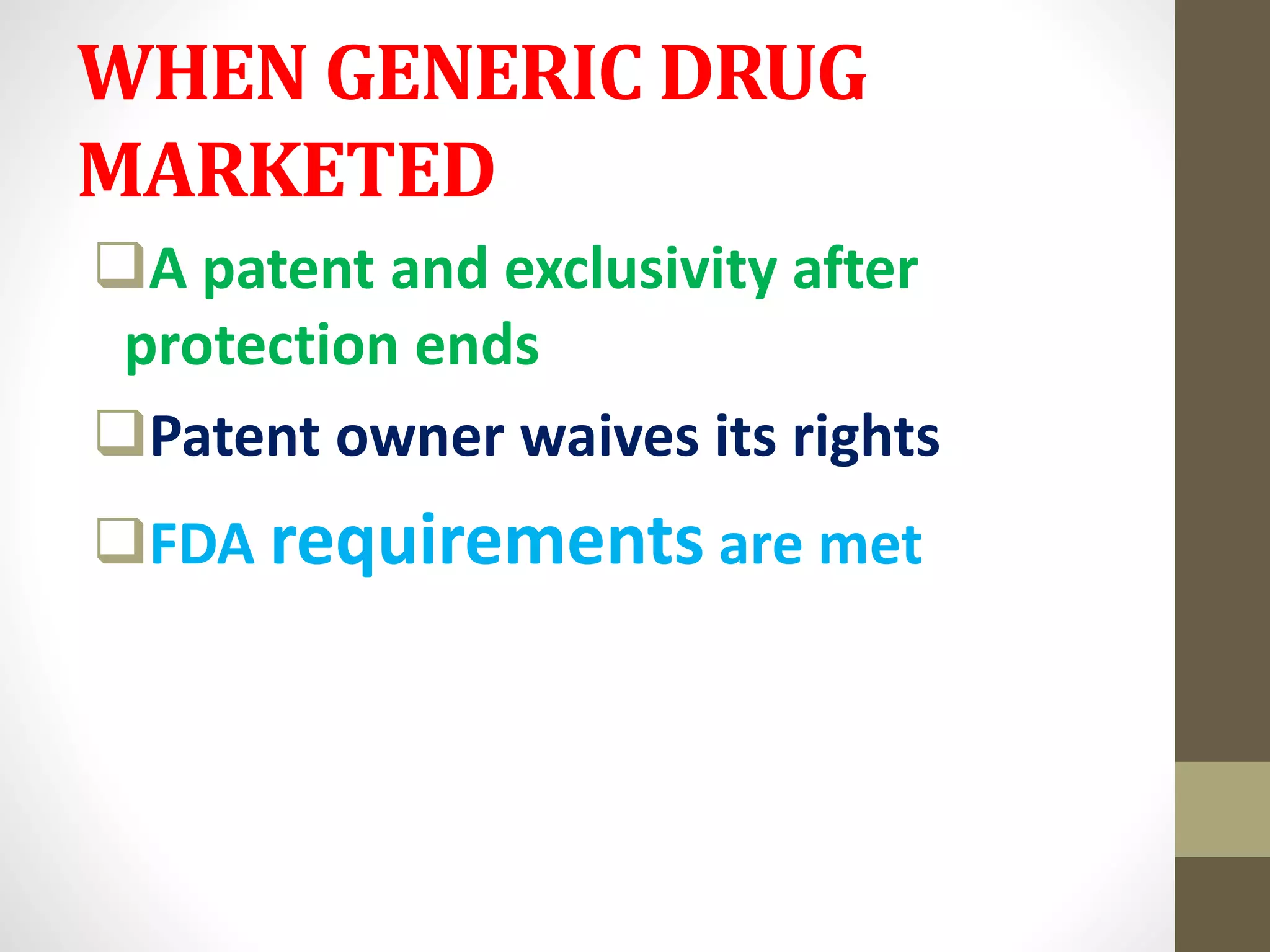
- Generic drugs are available after patents or marketing rights expire on brand name drugs. They maintain quality at affordable prices for critical diseases.