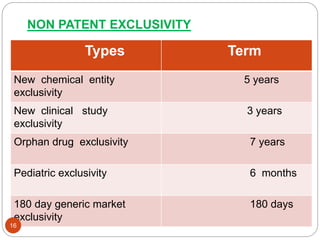
The Hatch-Waxman Act established an abbreviated approval pathway for generic drugs that relies on the safety and efficacy evidence of the branded reference drug. It aims to balance incentives for innovation and generic competition. The Act created ANDAs that allow generics to enter the market after patents and exclusivities expire. It also provides the branded drug up to 30 months to litigate patents against Paragraph IV ANDA challenges and restores some patent term lost during regulatory review.