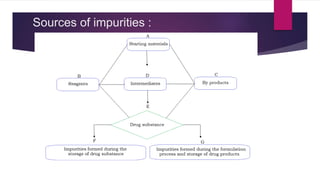
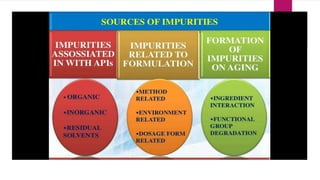
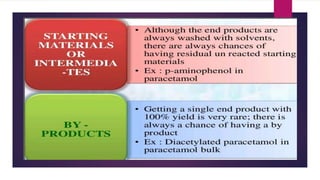
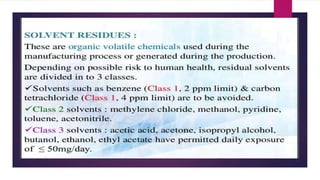
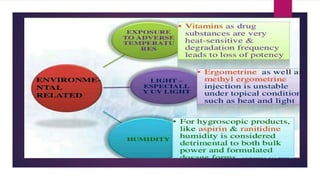
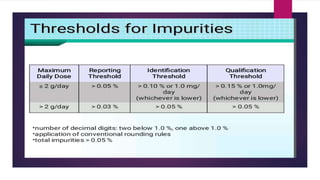
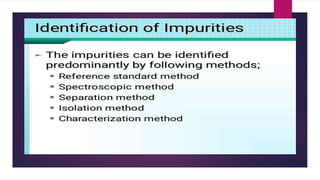
The document discusses impurity profiling in pharmaceuticals, emphasizing the significance of identifying and quantifying impurities that may adversely affect drug safety and efficacy. It outlines various sources of impurities, including raw materials and manufacturing processes, and describes methods for isolation and characterization of these impurities. Additionally, it provides references to guidelines and studies related to pharmaceutical impurities.