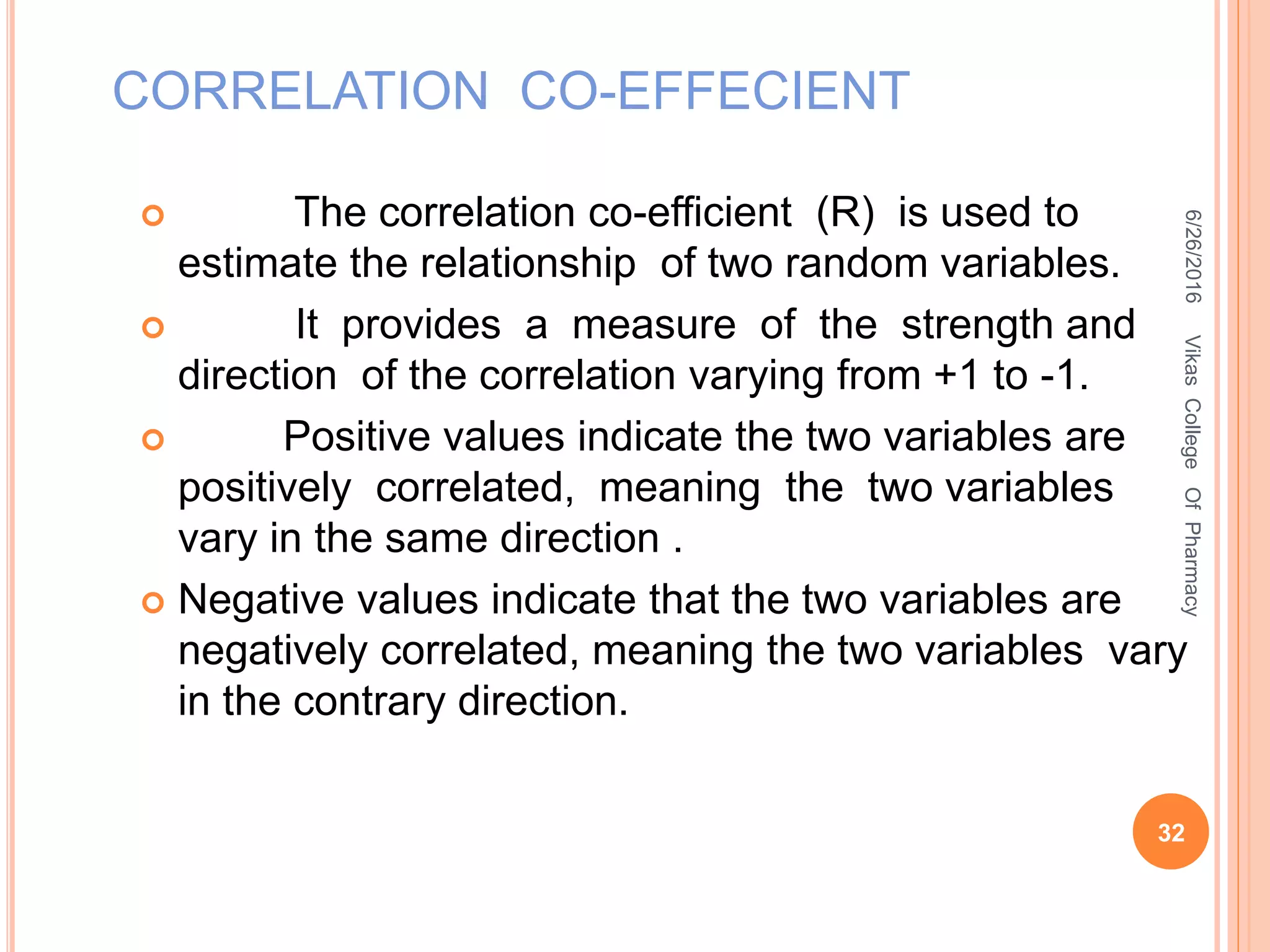
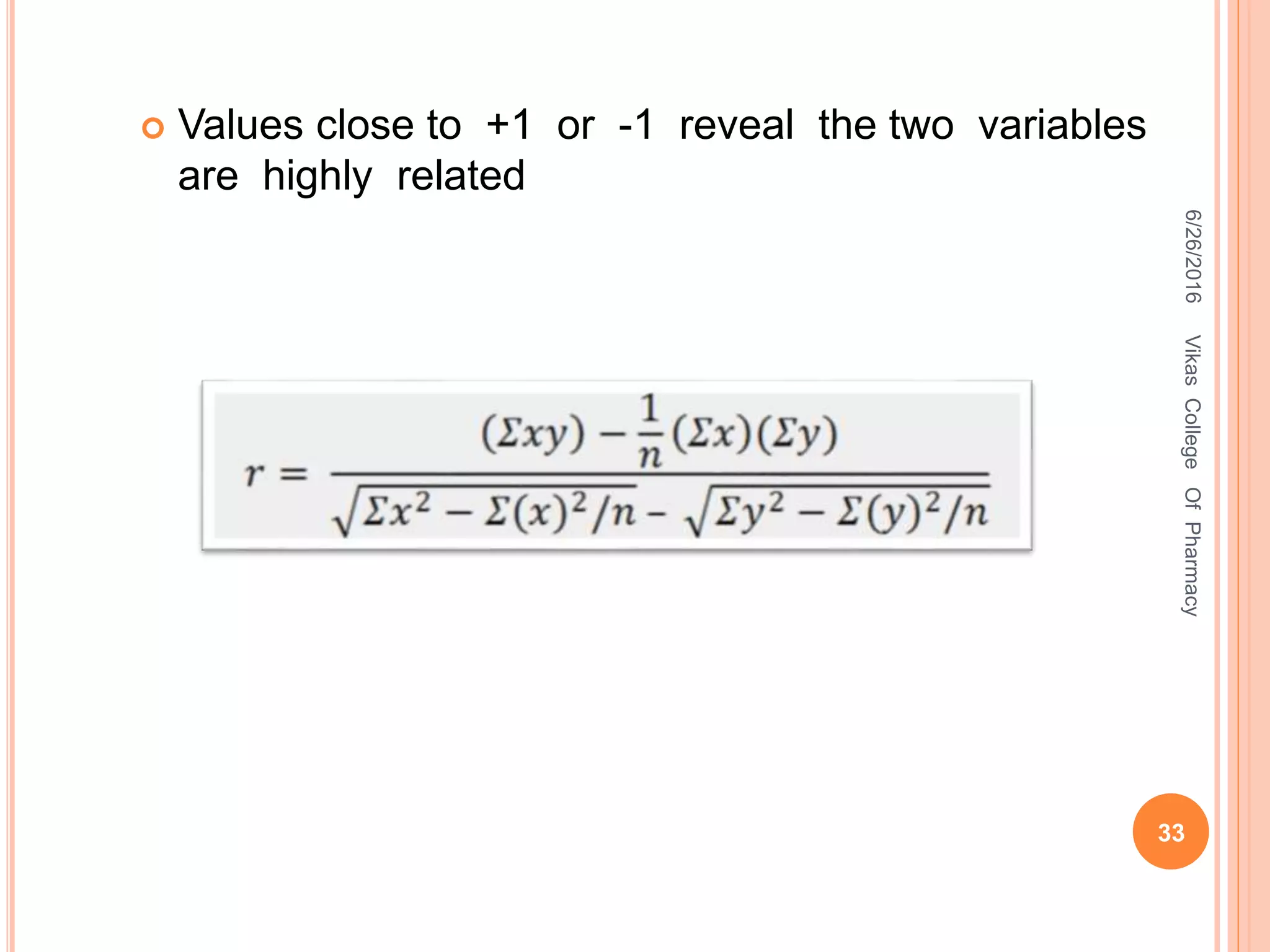
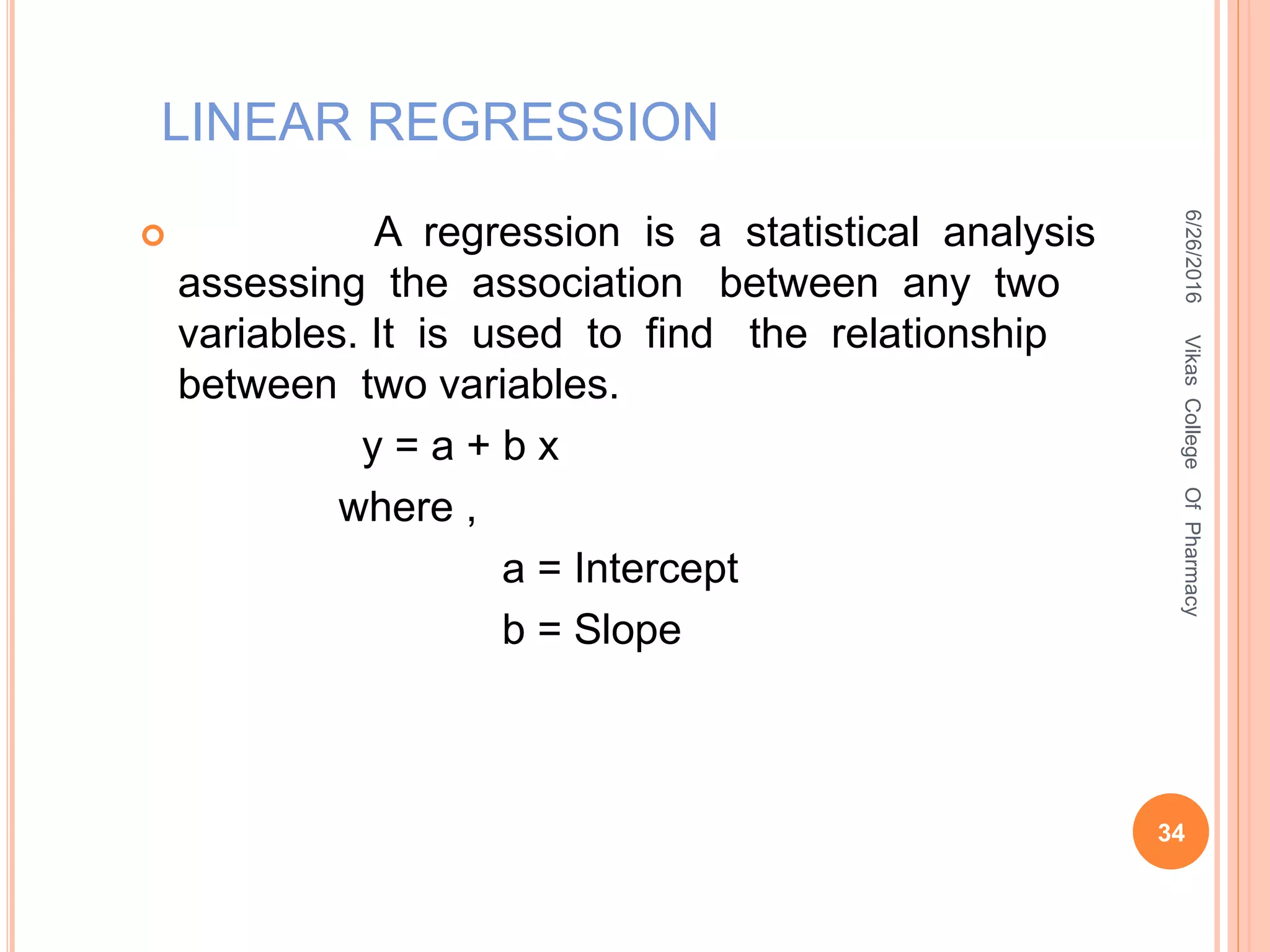
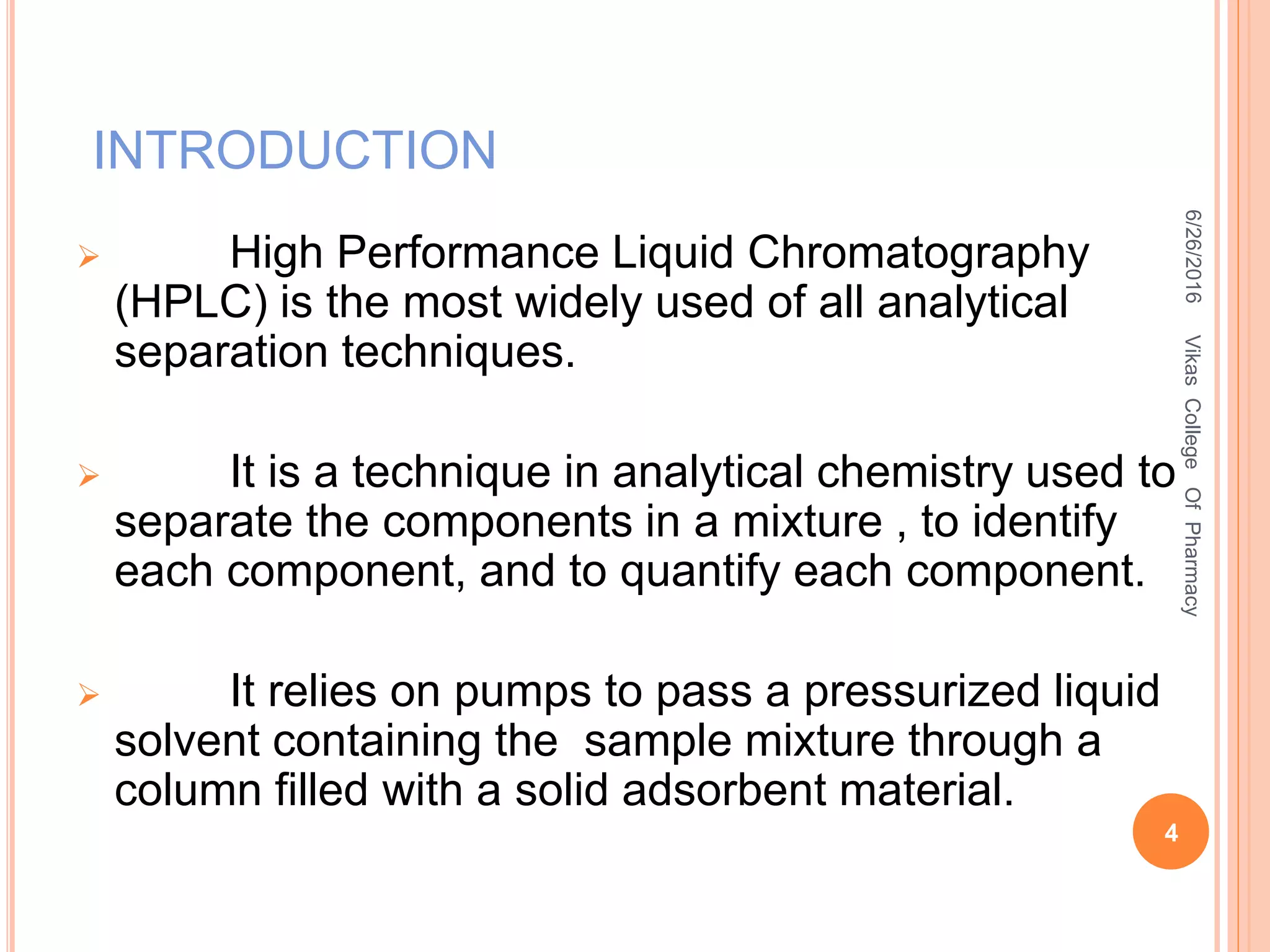
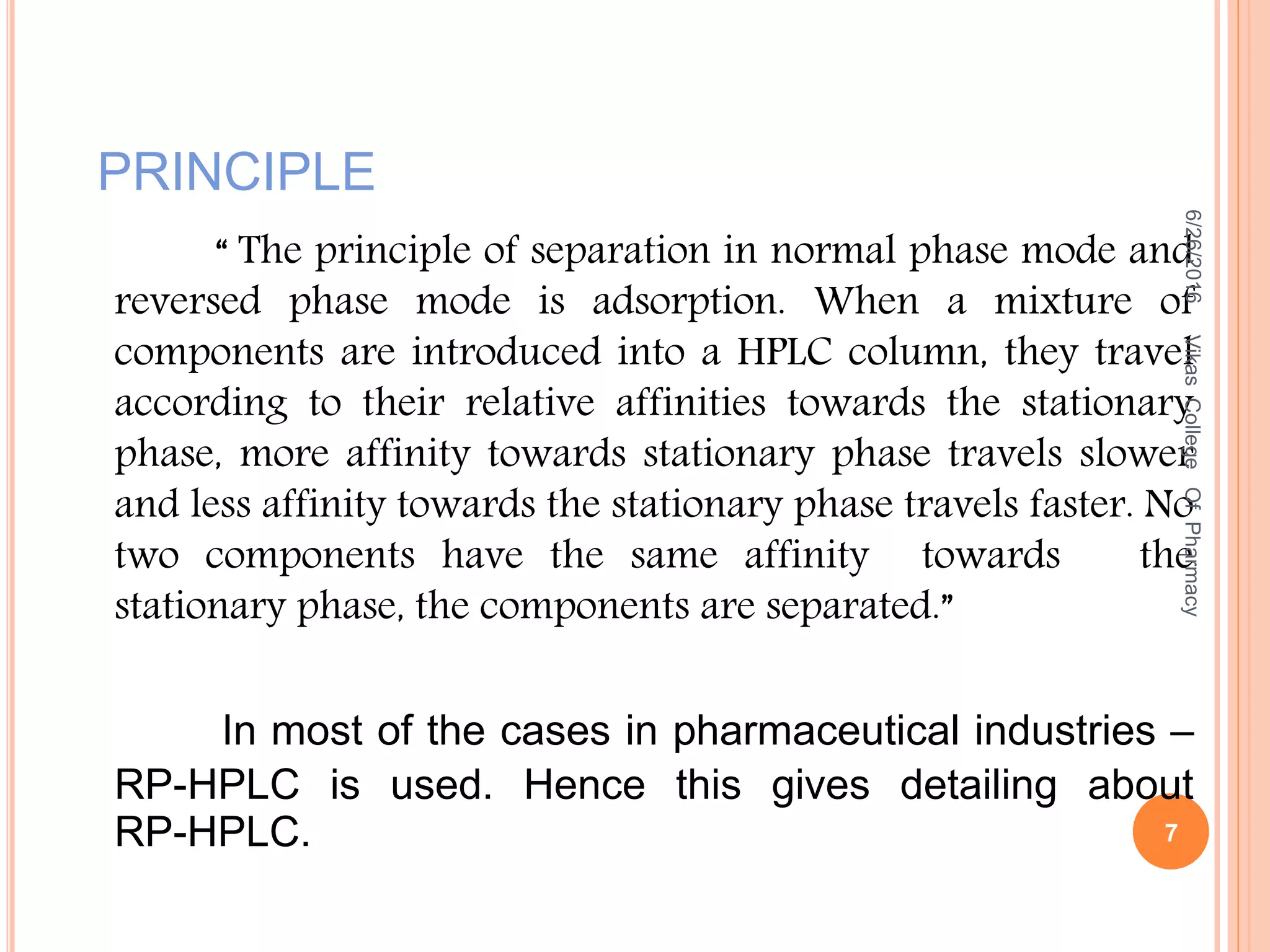
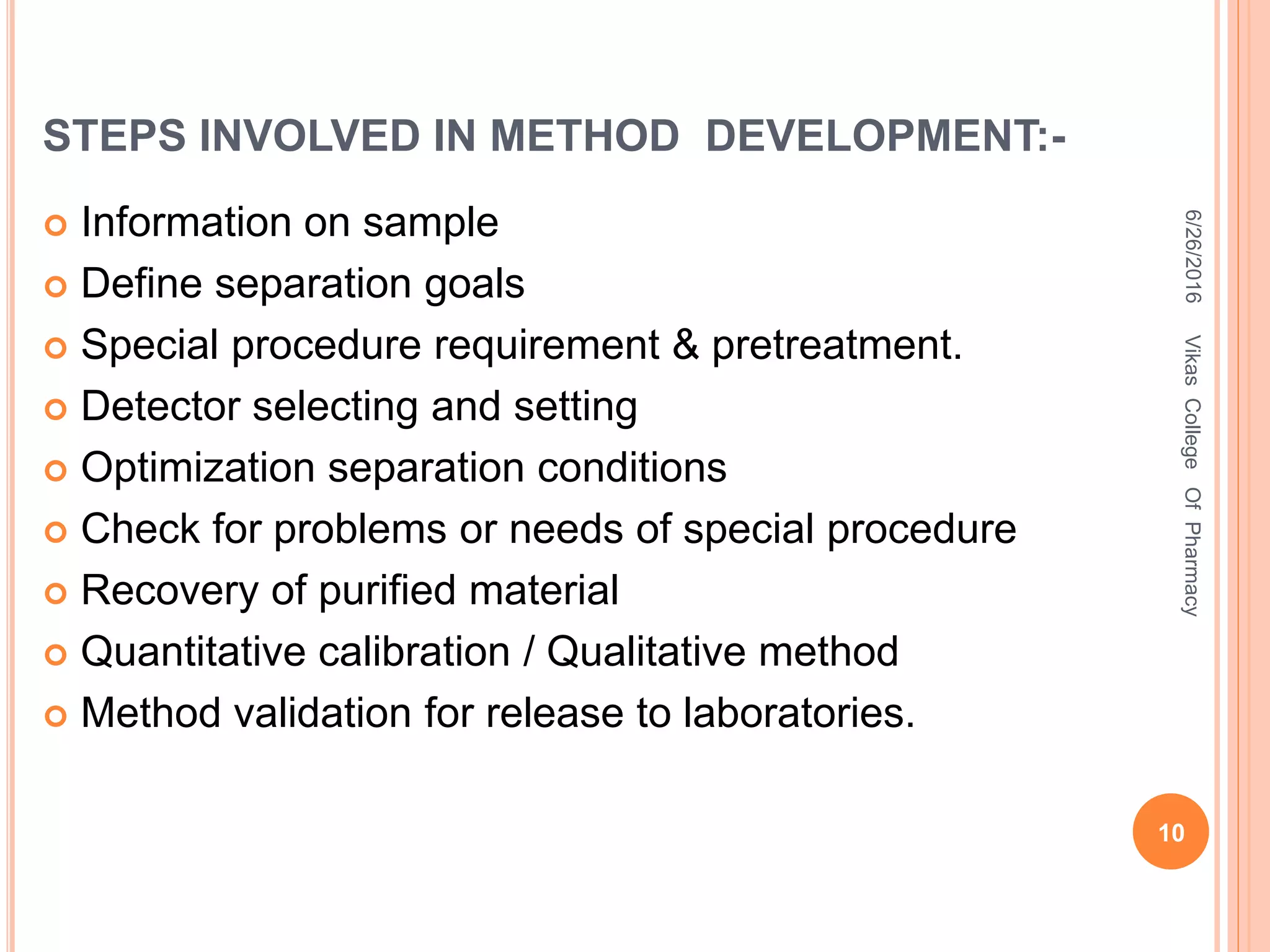
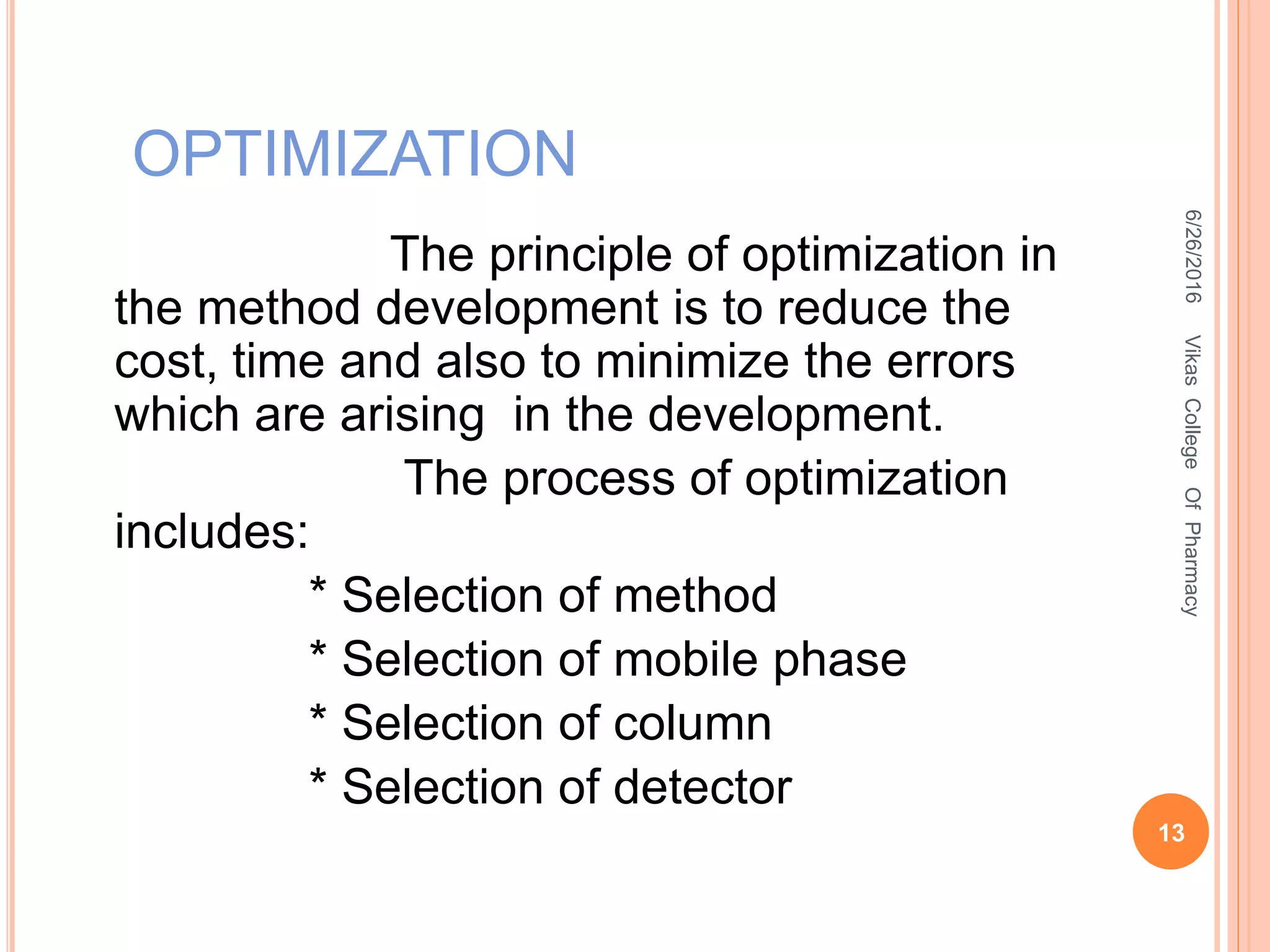
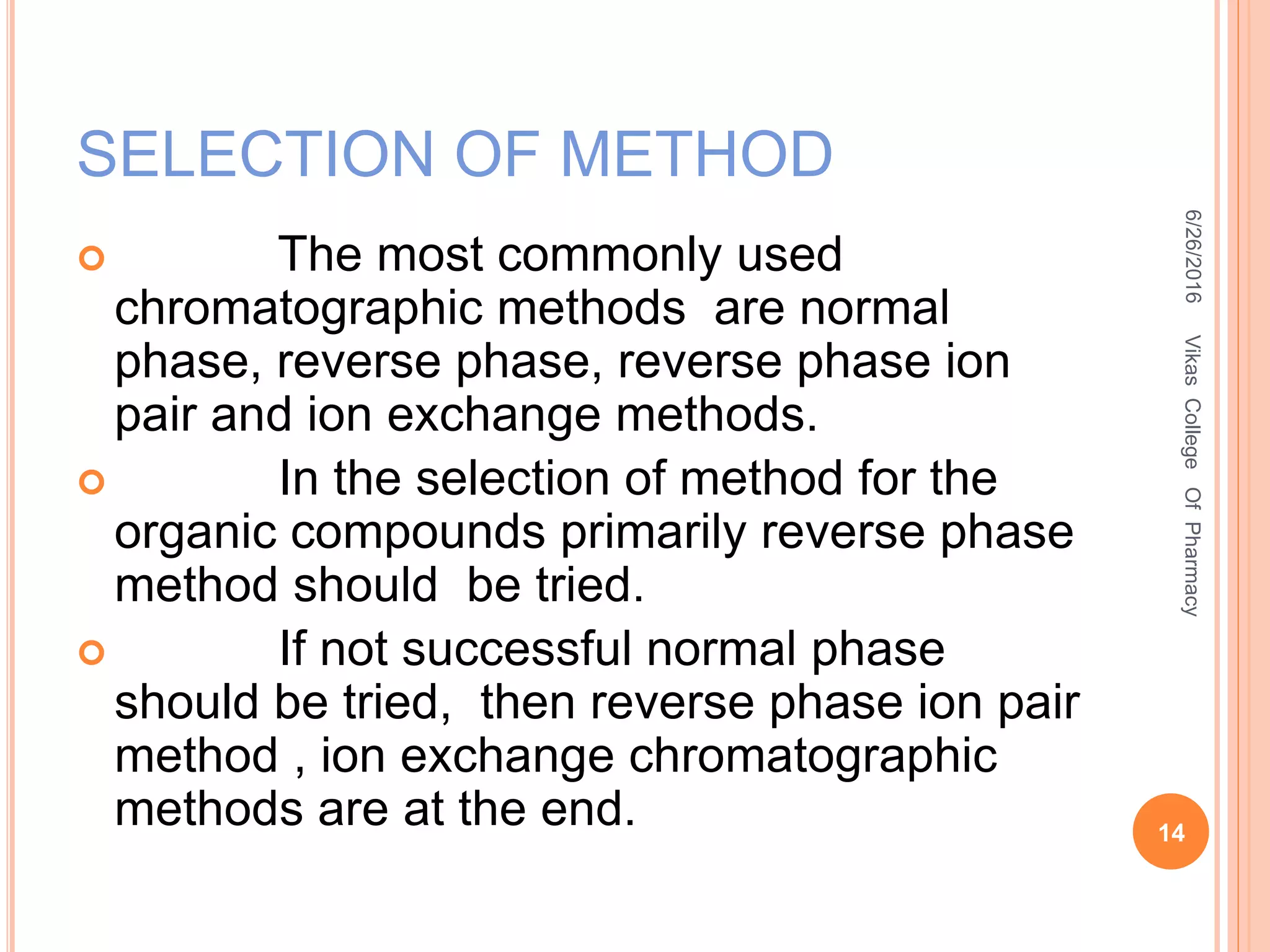
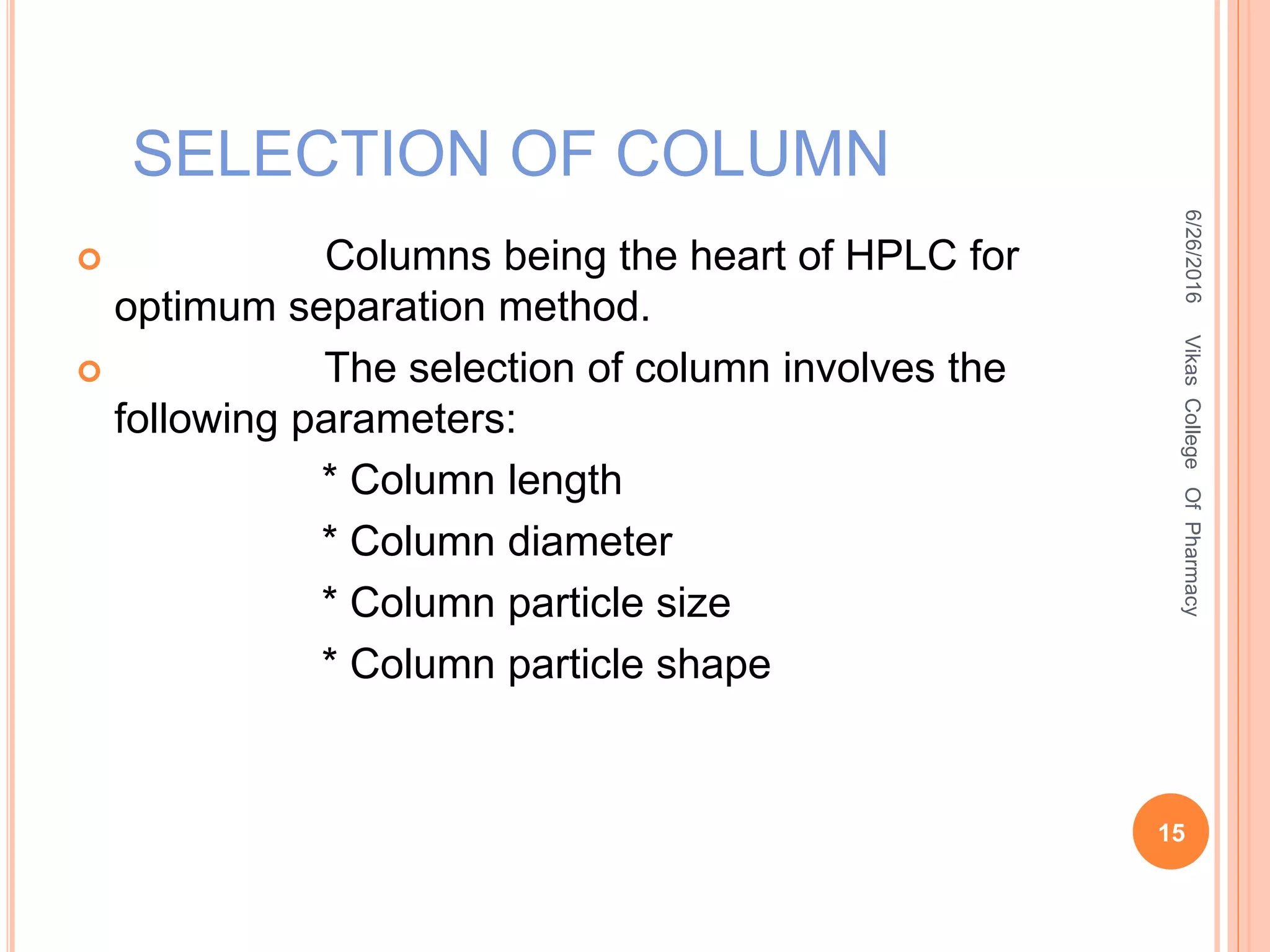
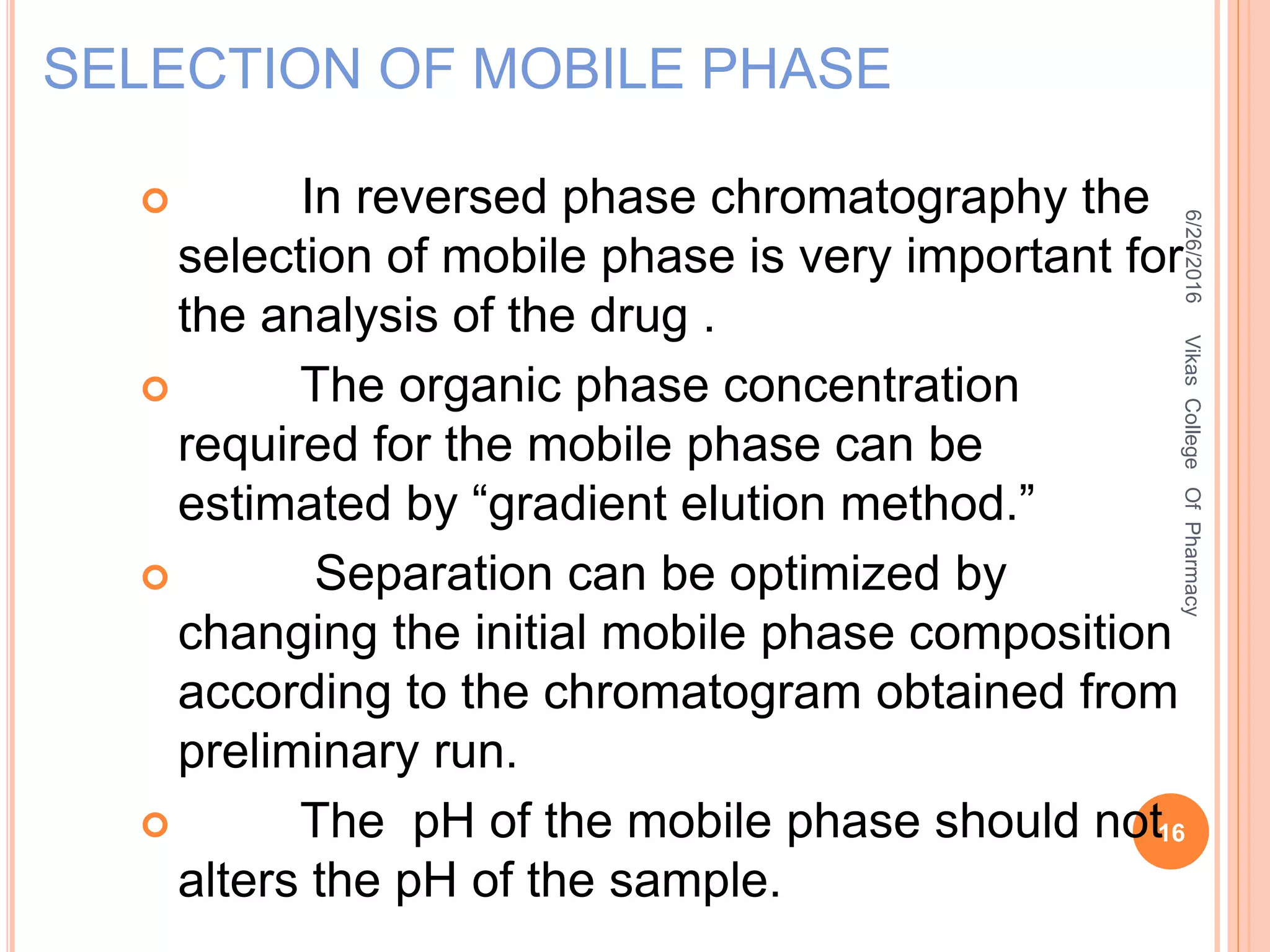
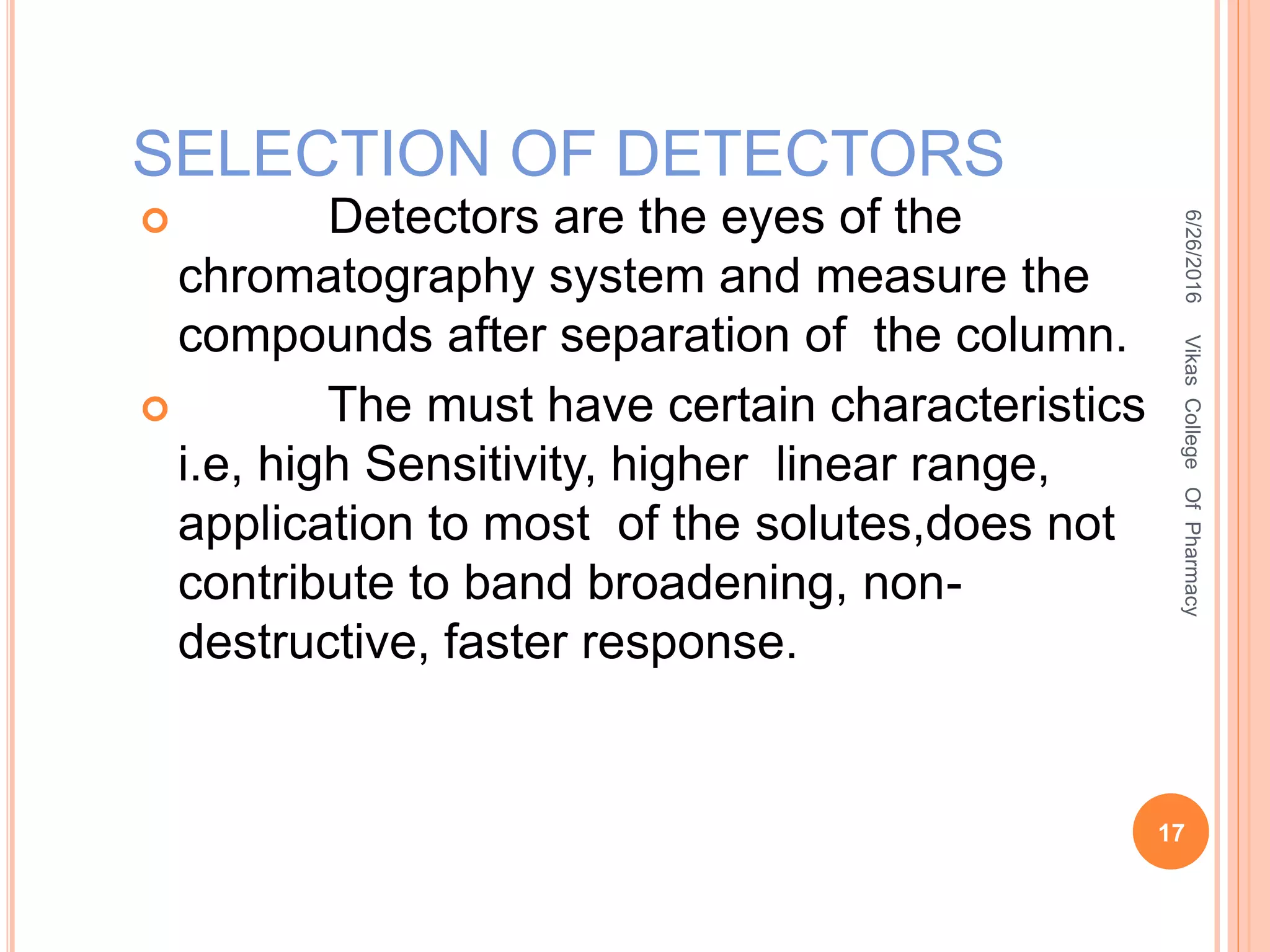
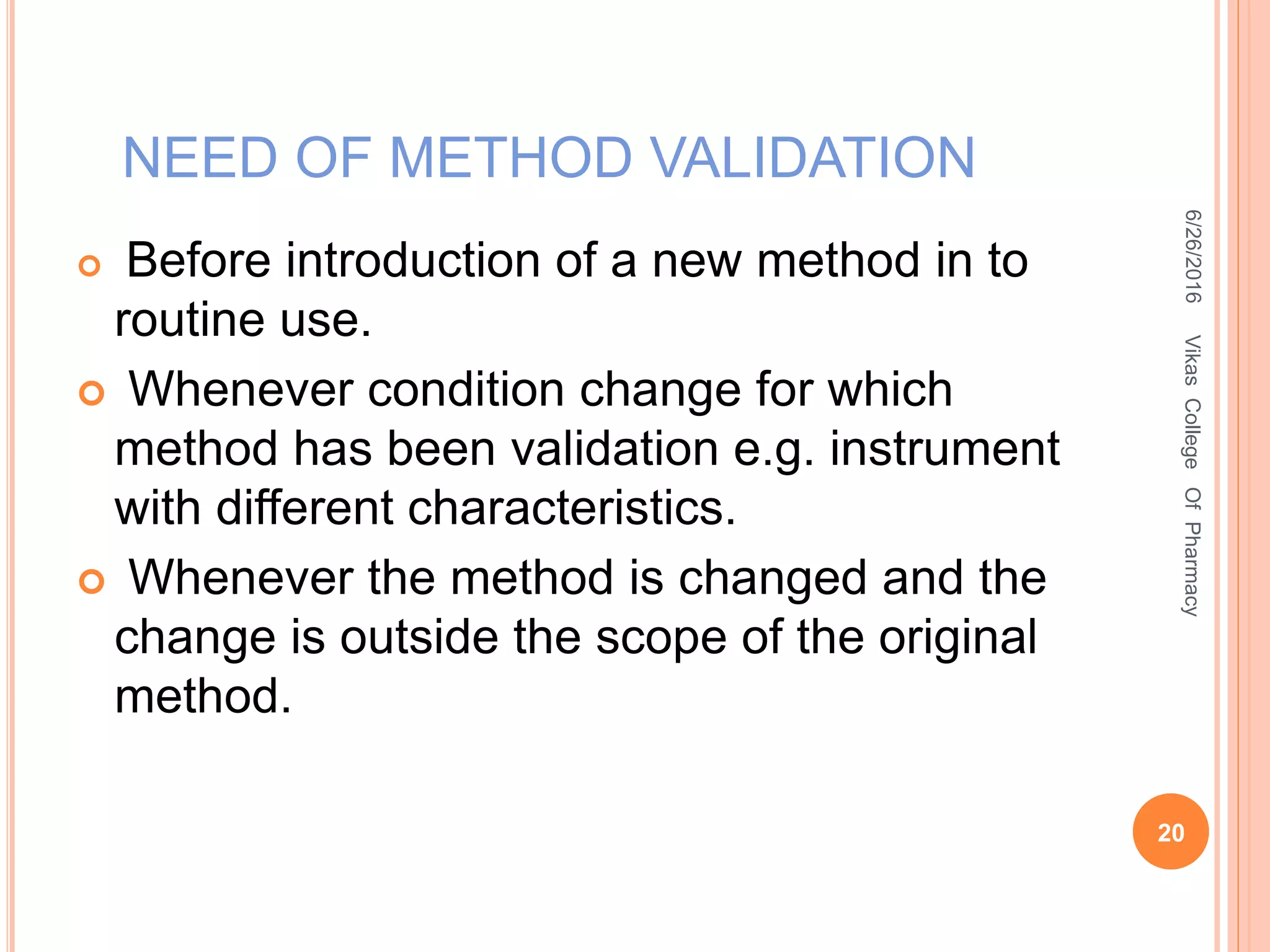
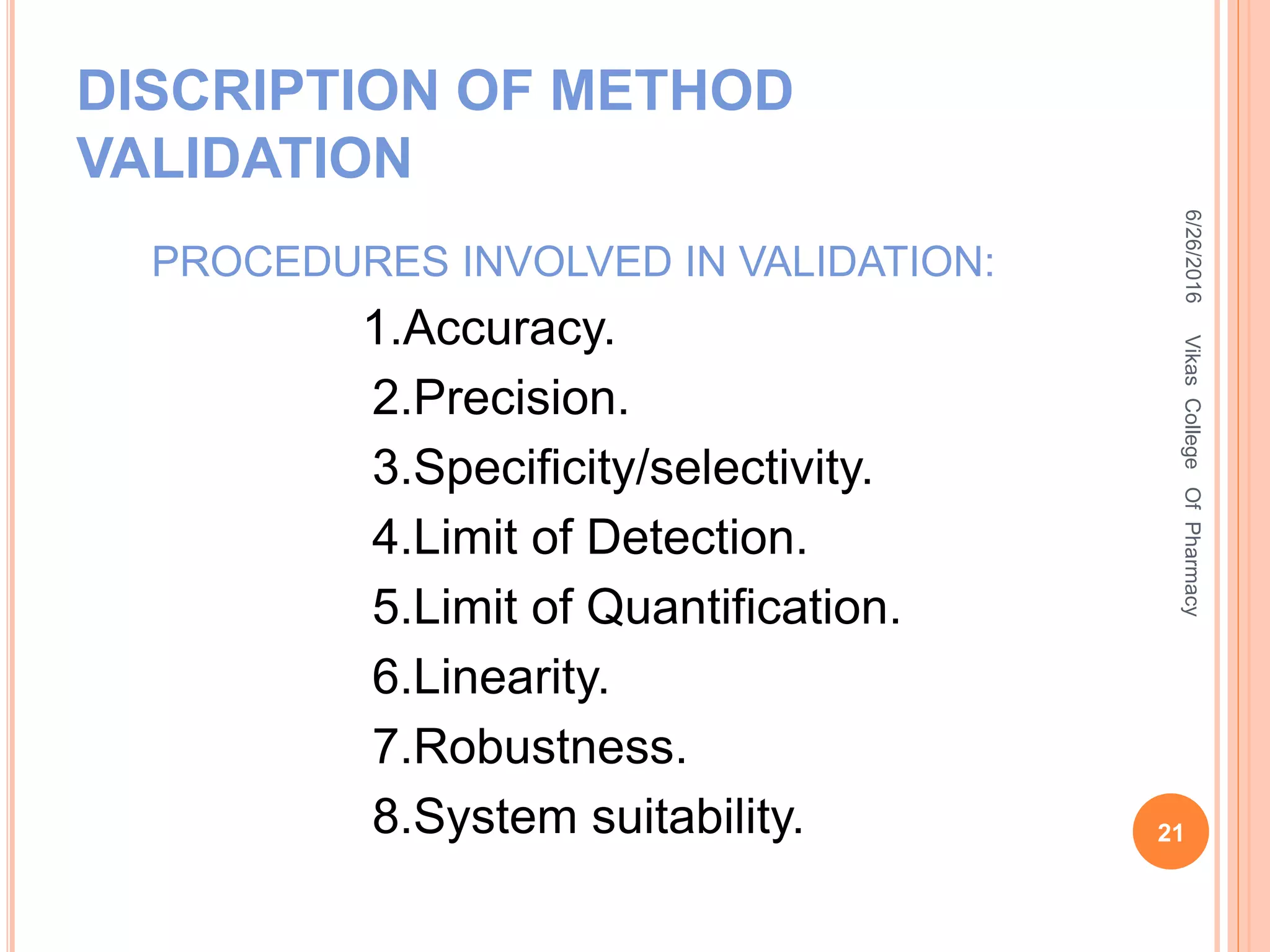
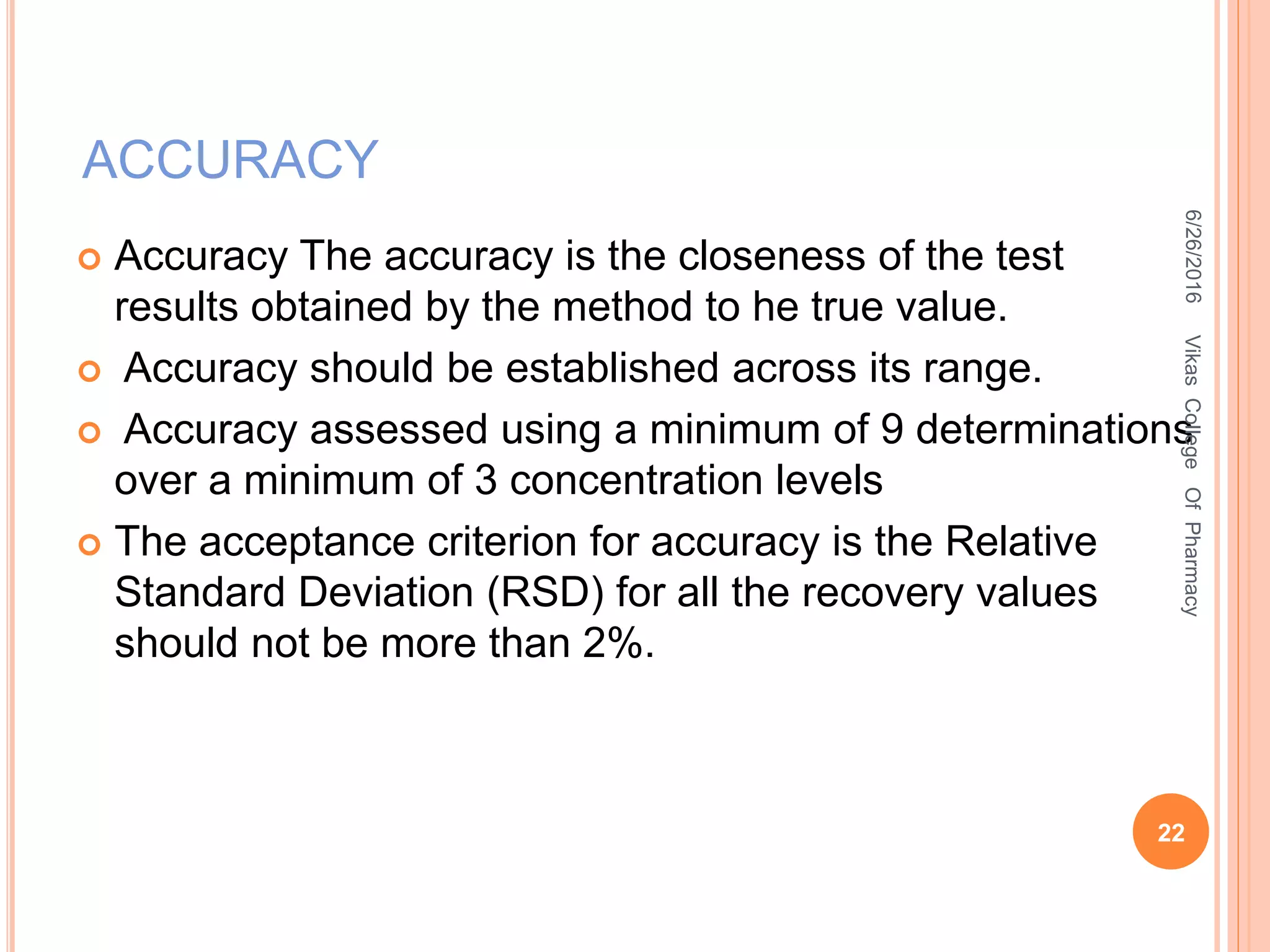
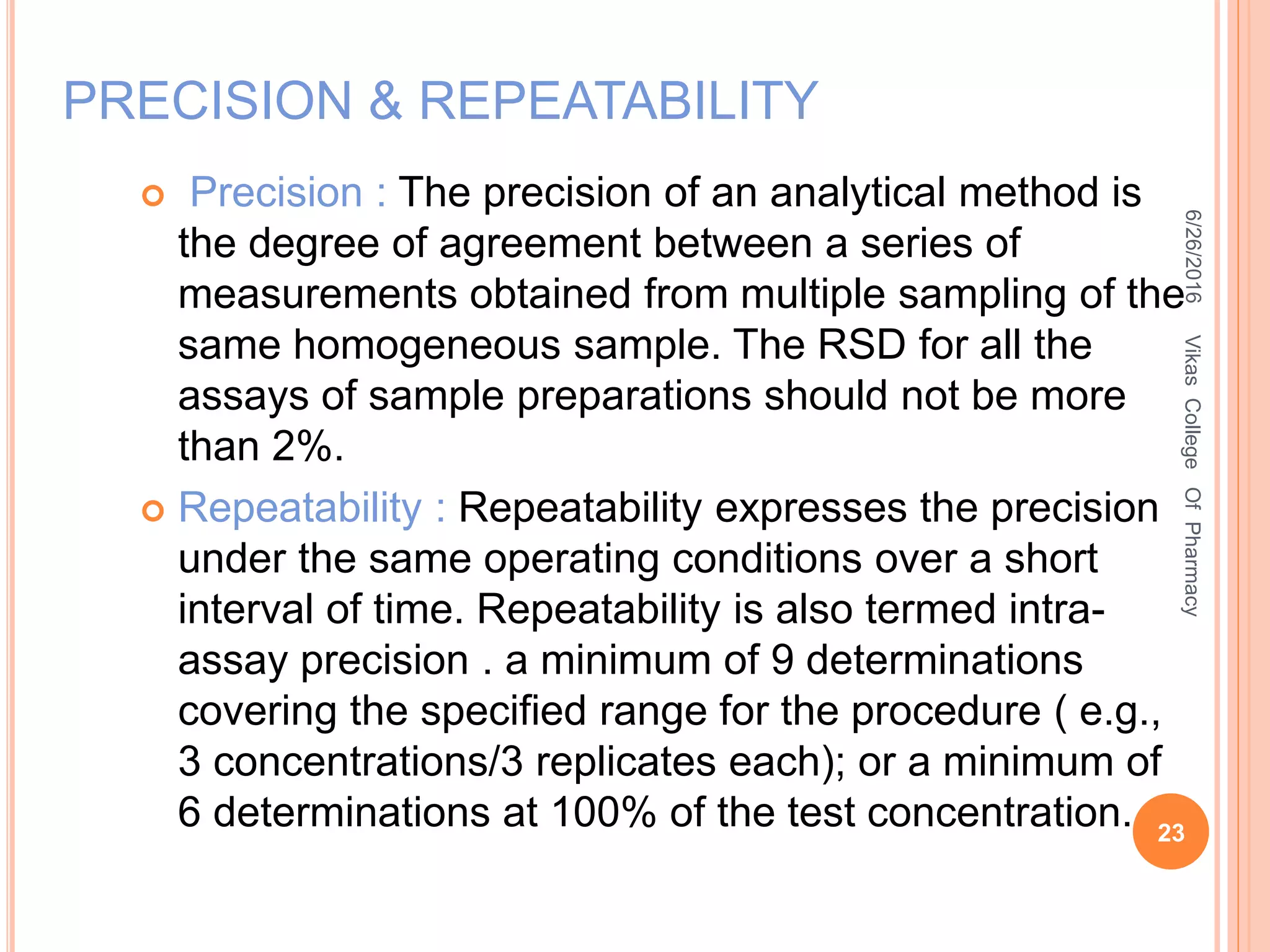
This document describes the development and validation of an HPLC method for estimating drugs. It discusses the principles of HPLC, steps in method development including selecting the method, column, mobile phase and detector. Method validation parameters like accuracy, precision, specificity, linearity and robustness are also summarized. The document provides details on the optimization process and validation procedures to ensure the method is suitable for its intended use.












![STANDARD DEVIATION
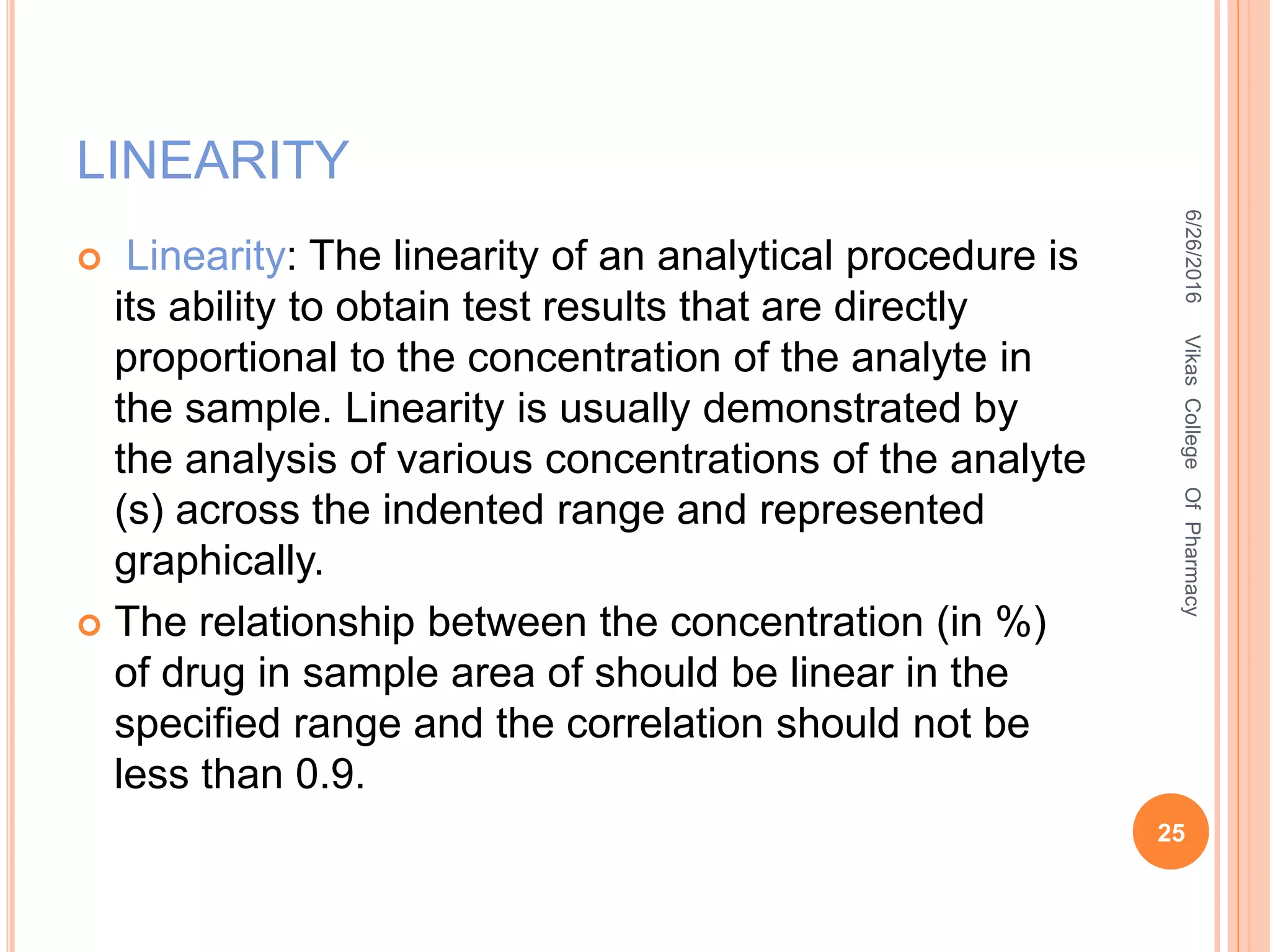
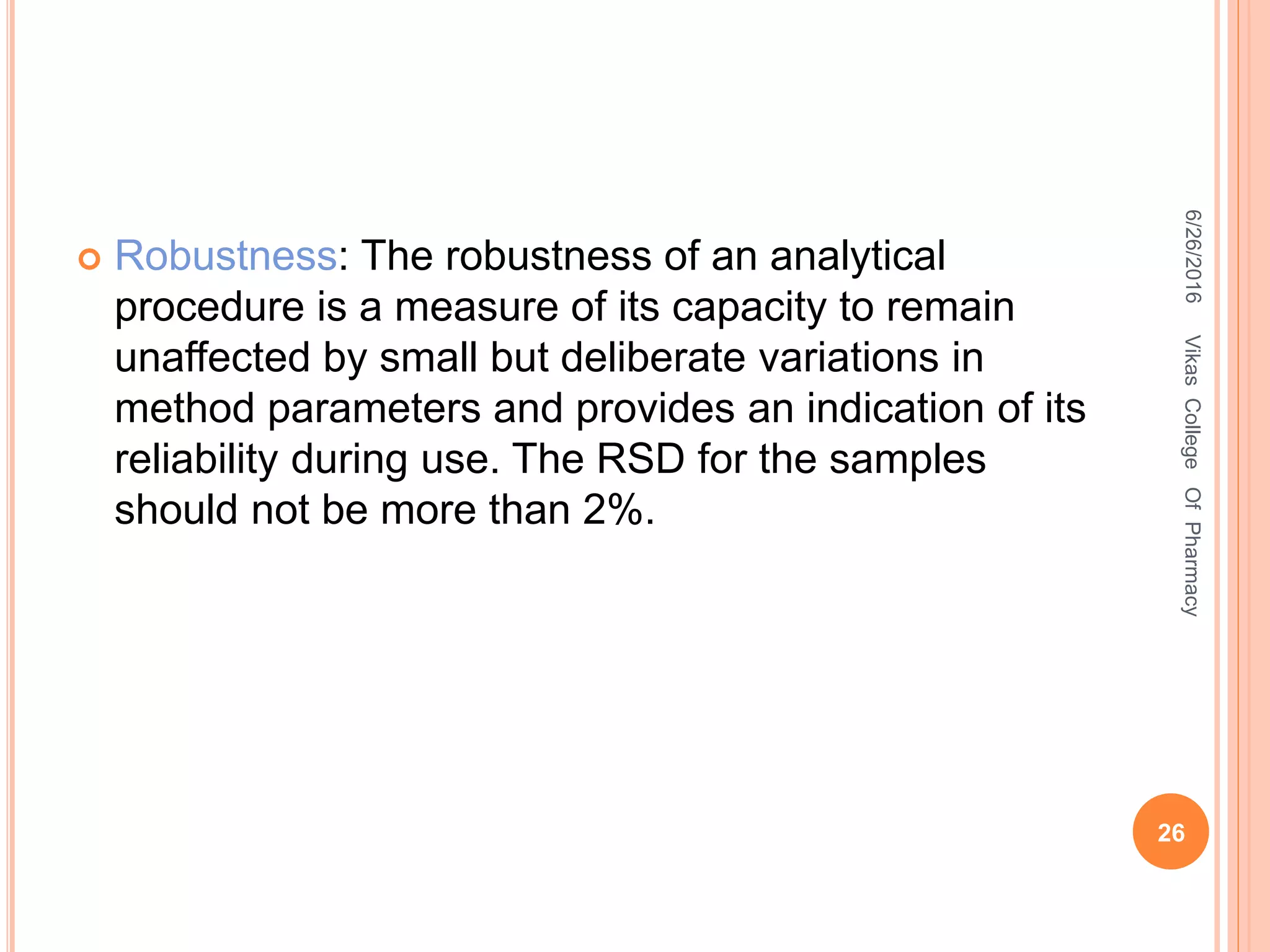
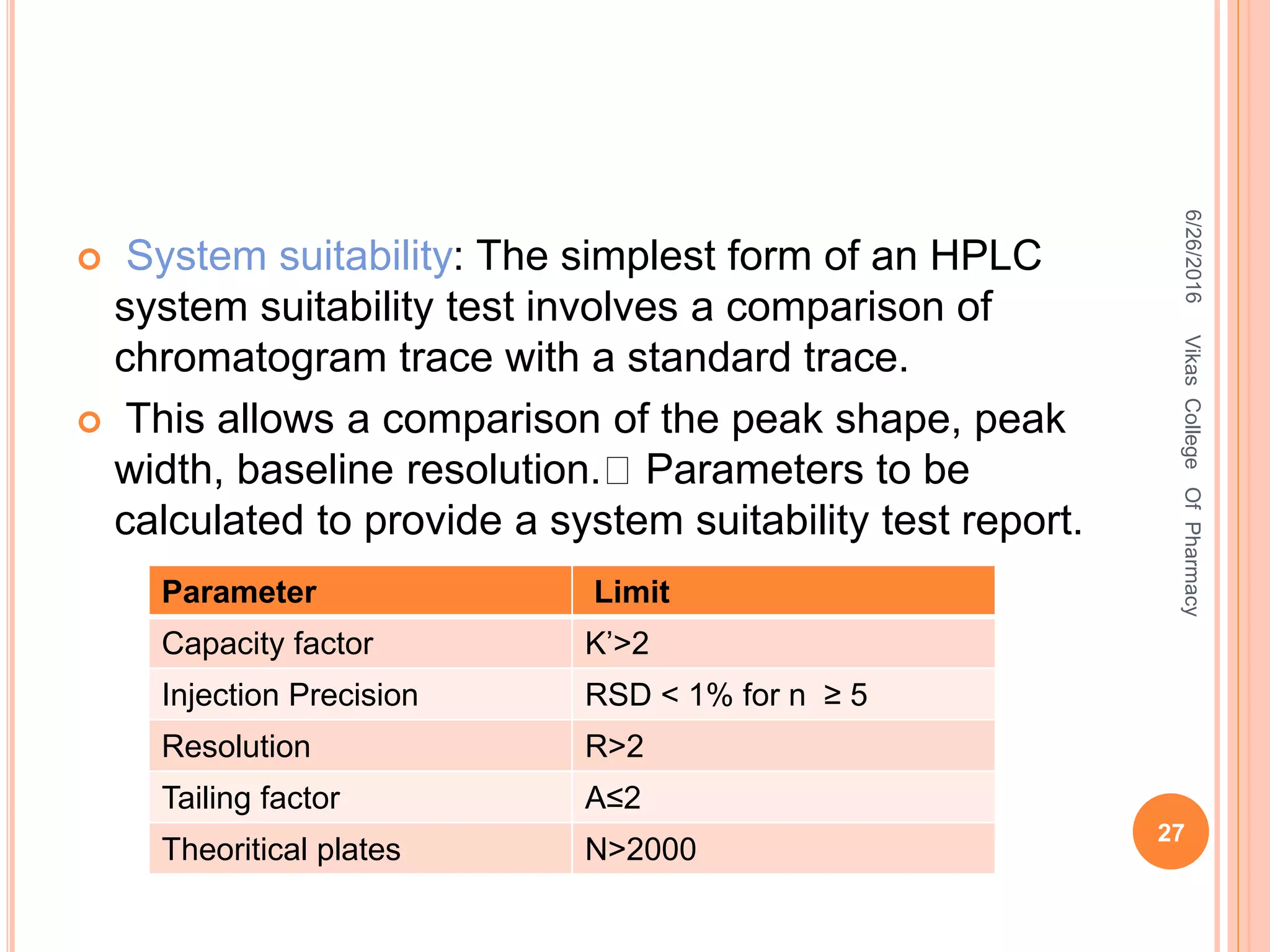
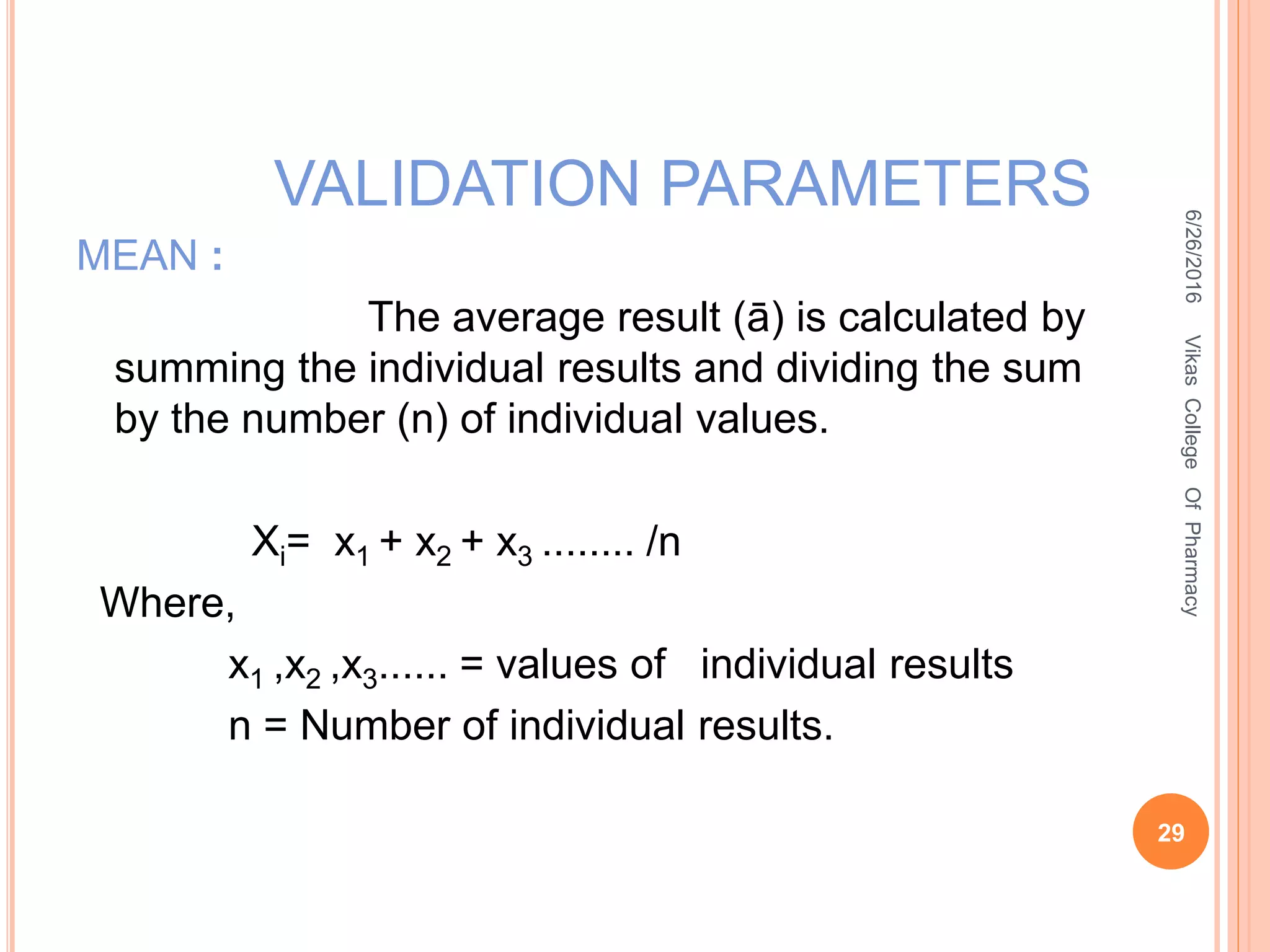
It is the root mean square deviation of values from
their average
SD = [Σ (X- Xi) /n-1]1/2
Where,
Σ = sum of observations
Xi = mean
X = individual observation value
(X- Xi) = deviation of a value from mean
n =number of observations
6/26/2016
30
VikasCollegeOfPharmacy](https://image.slidesharecdn.com/mdmvmr-160626073720/75/HPLC-Method-Development-Method-Validation-mr-s-30-2048.jpg)