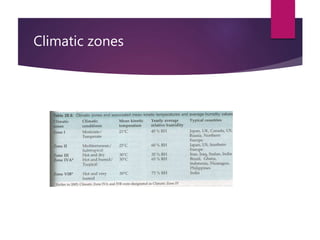
The document discusses the importance of stability and stability testing of pharmaceutical products, which ensures the retention of quality, safety, and efficacy throughout their shelf life. It outlines guidelines for stability testing protocols, which include assessing environmental effects on drug substances, conducting stress tests, and following specified storage conditions. Additionally, it emphasizes the need for long-term commitment to stability studies and proper labeling based on stability evaluations.