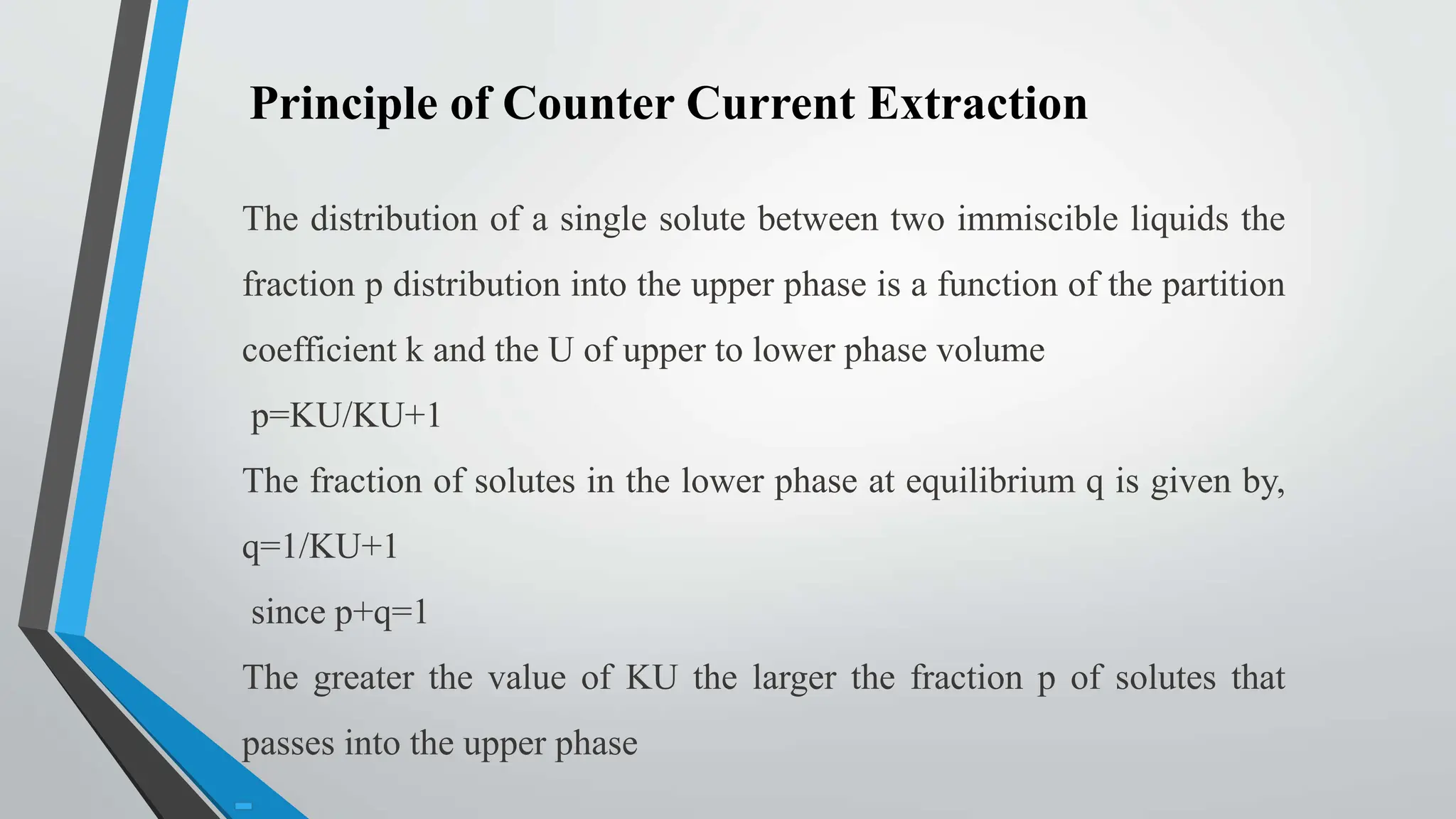
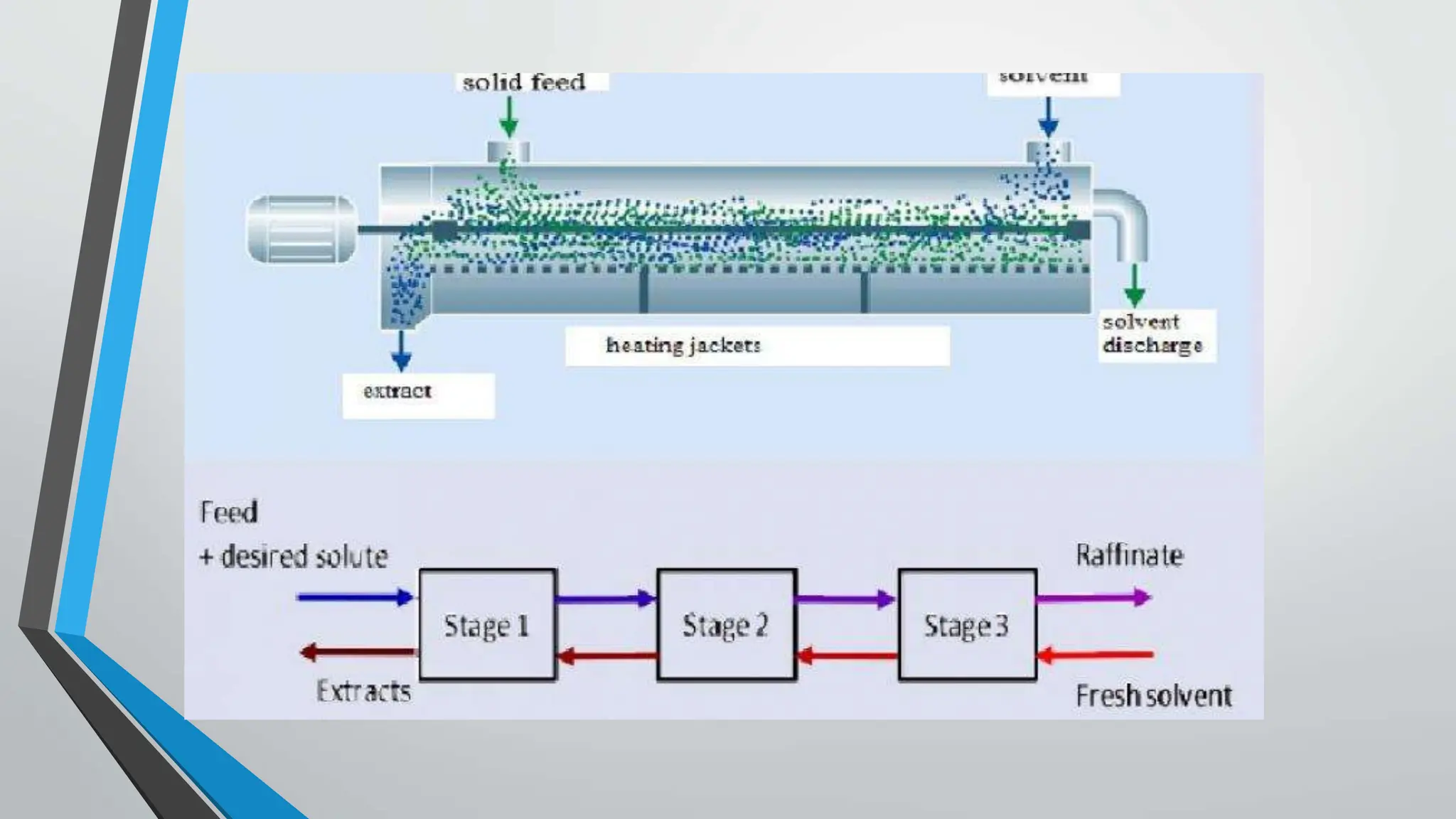
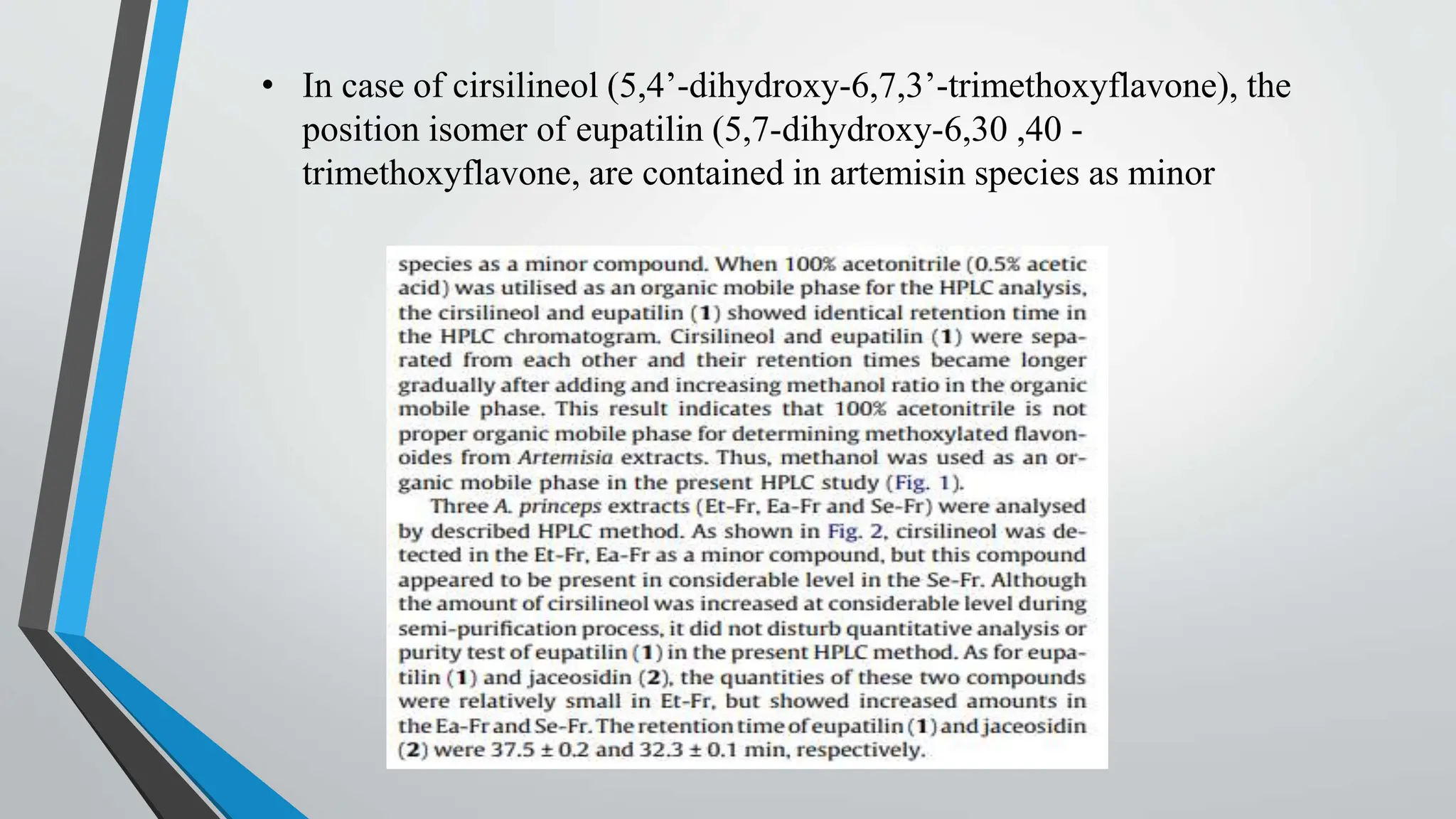
Counter current extraction is a multi-stage liquid-liquid extraction method that separates components based on solubility in two immiscible liquids flowing in opposite directions. Utilizing Craig apparatus, it efficiently isolates substances such as antibiotics and chemical compounds while preventing thermal degradation. The study successfully separated anti-ulcer compounds eupatilin and jaceosidin from artemisia extracts using a two-phase solvent system, demonstrating superior yield and purity compared to traditional chromatography.