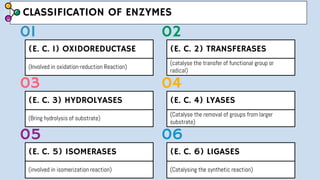
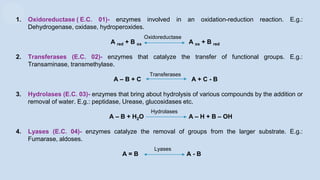
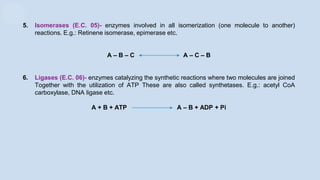
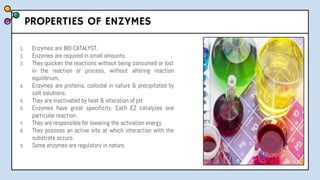
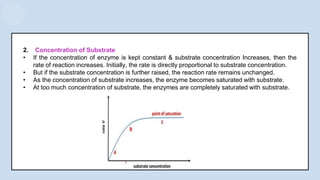
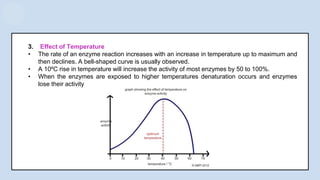
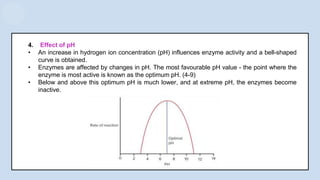
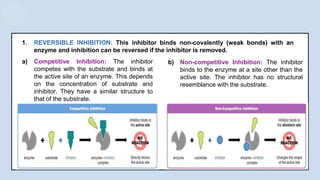
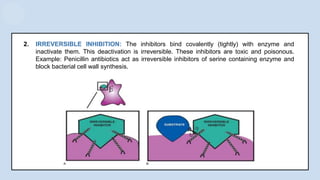
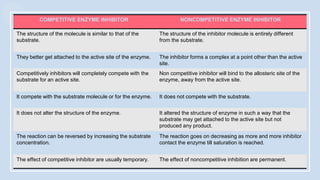
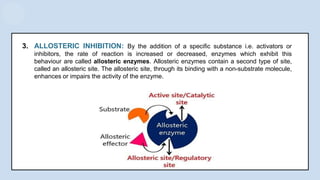
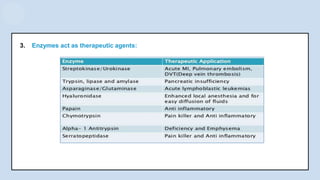
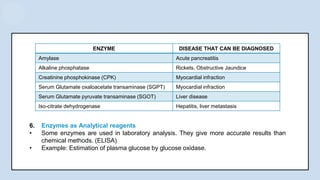
The document provides an overview of enzymes, highlighting their role as biological catalysts that accelerate biochemical reactions with specificity and efficiency. It discusses their structure, classification, mechanisms of action, and factors influencing enzyme activity, along with the impact of inhibitors. Additionally, it covers the therapeutic, diagnostic, and industrial applications of enzymes, demonstrating their importance in various fields.