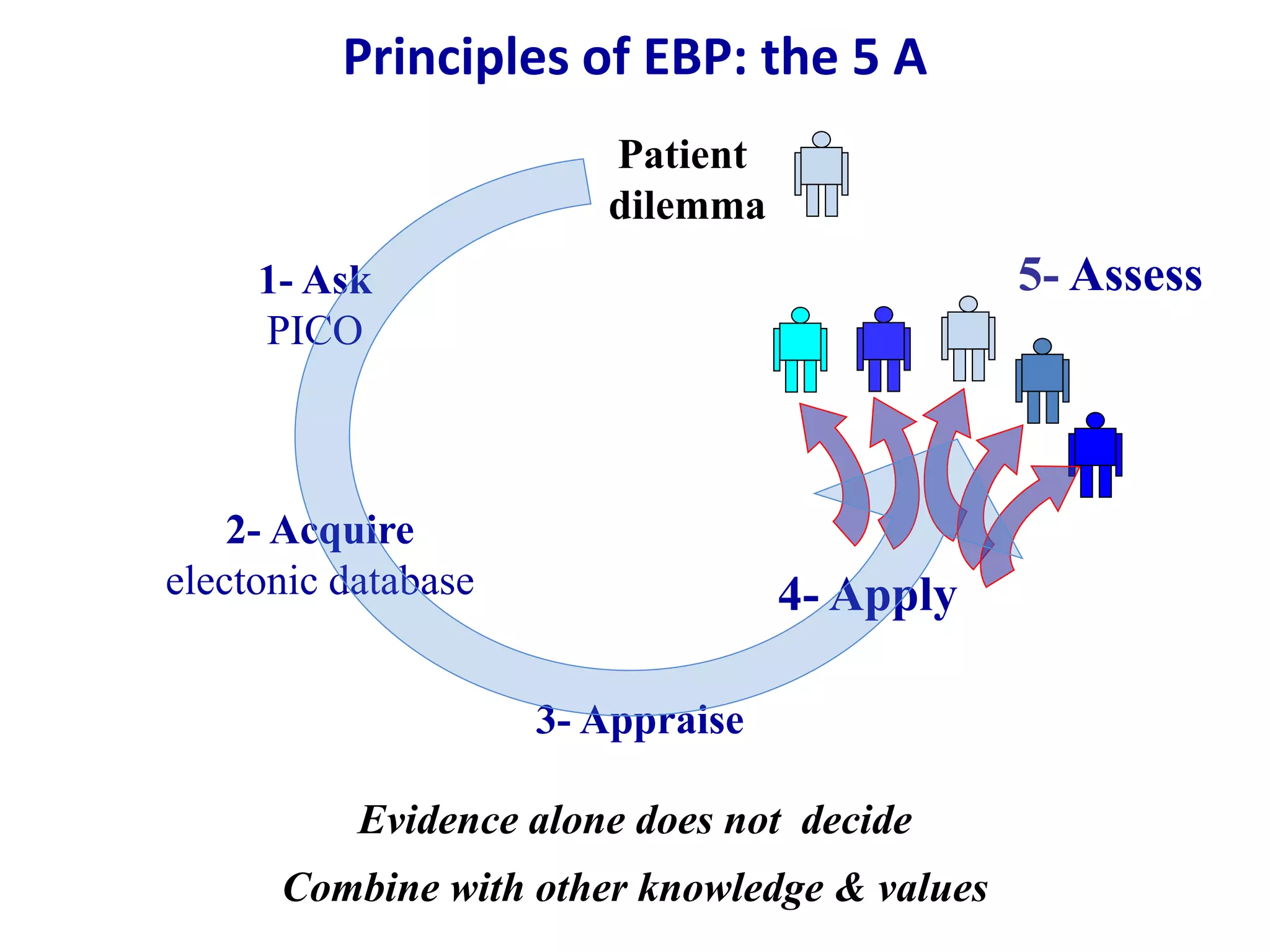
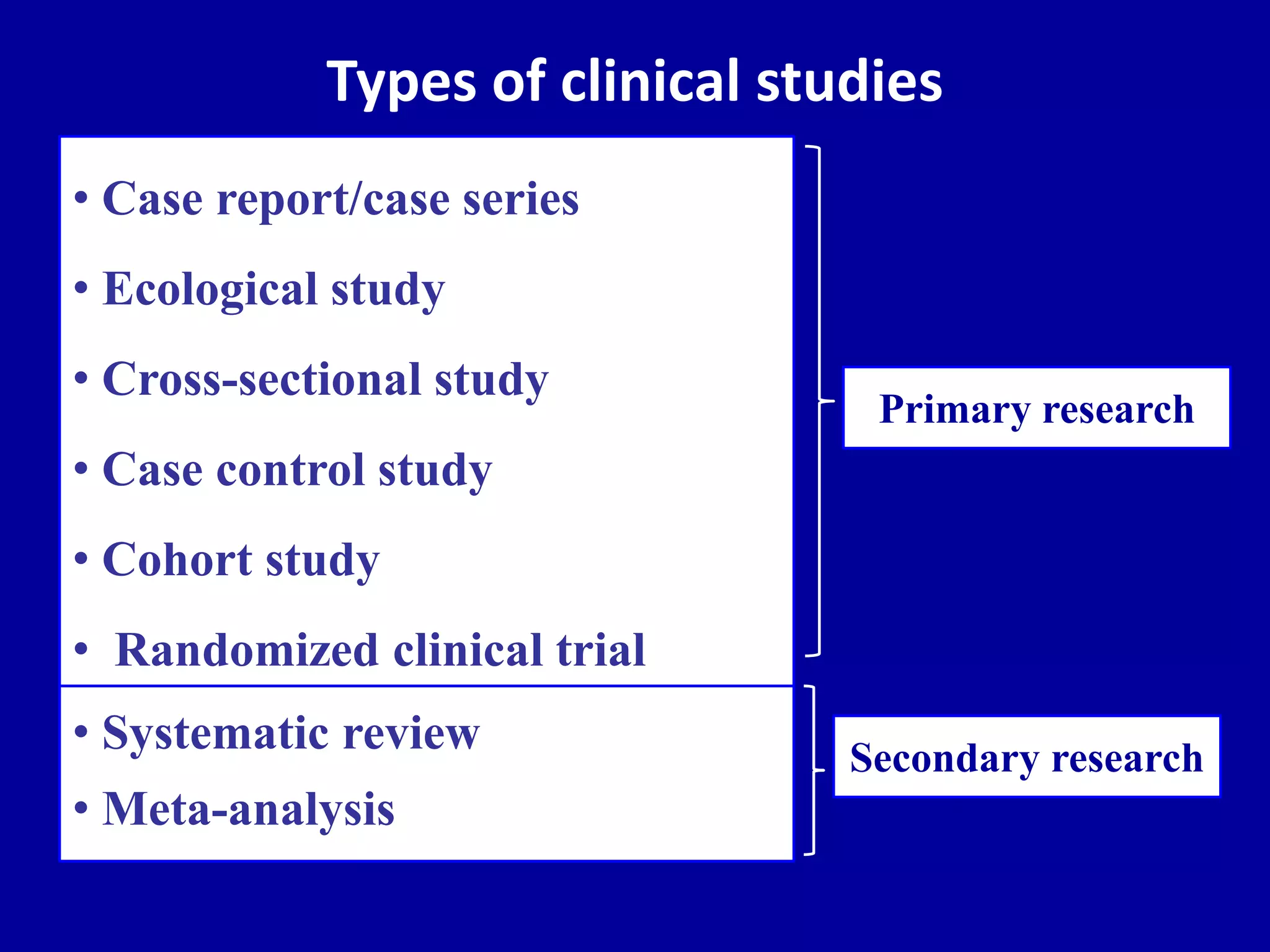
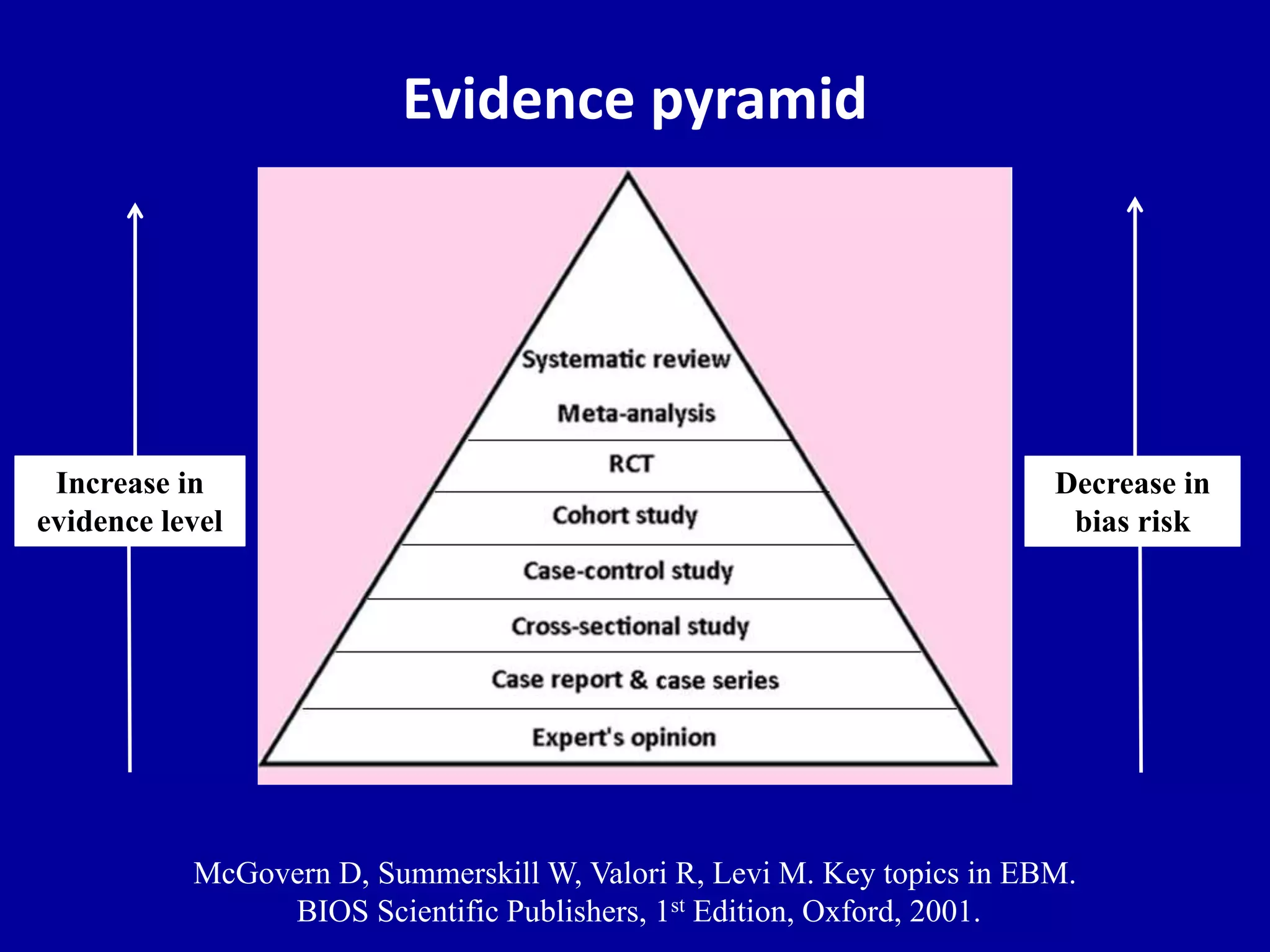
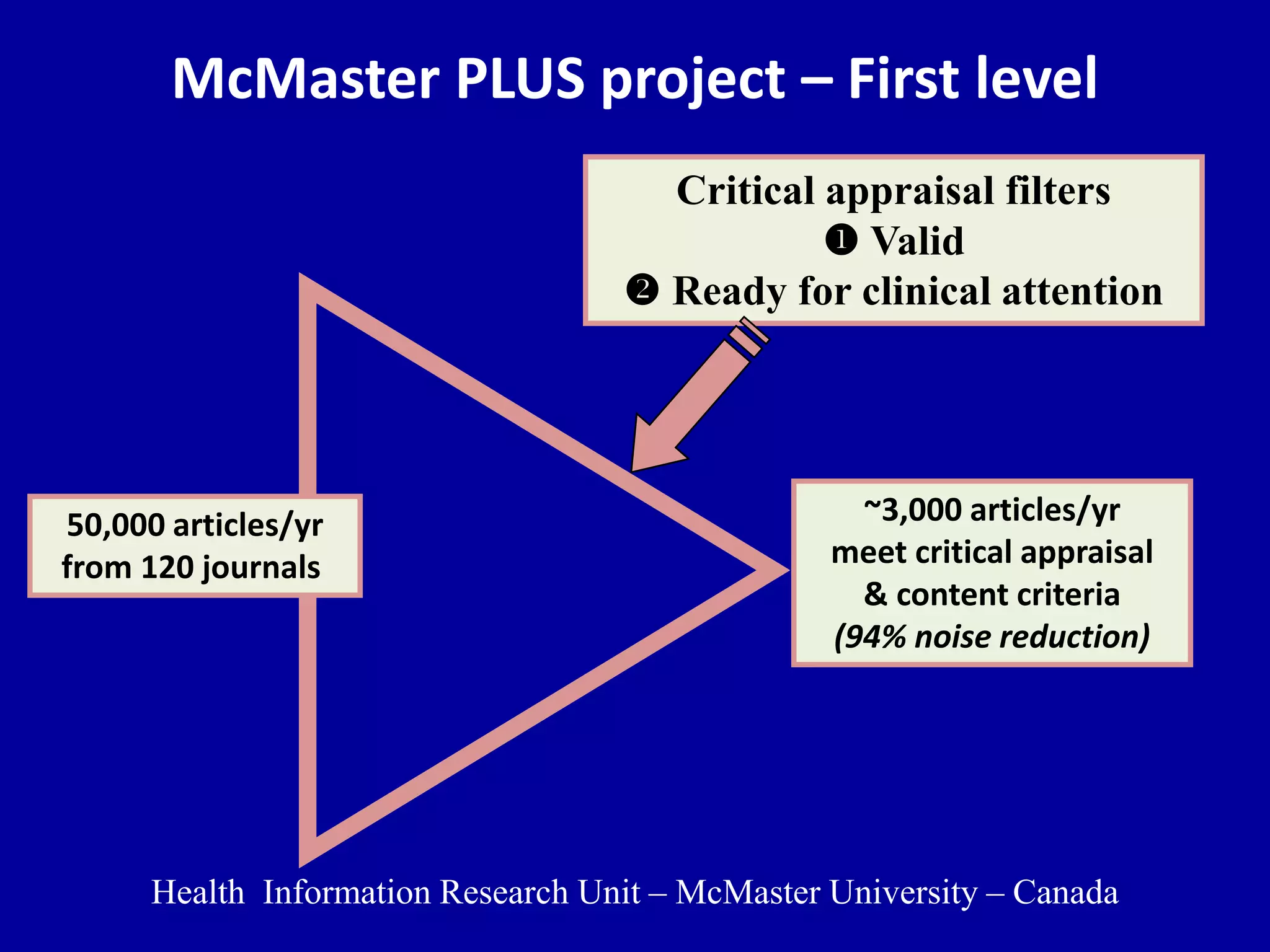
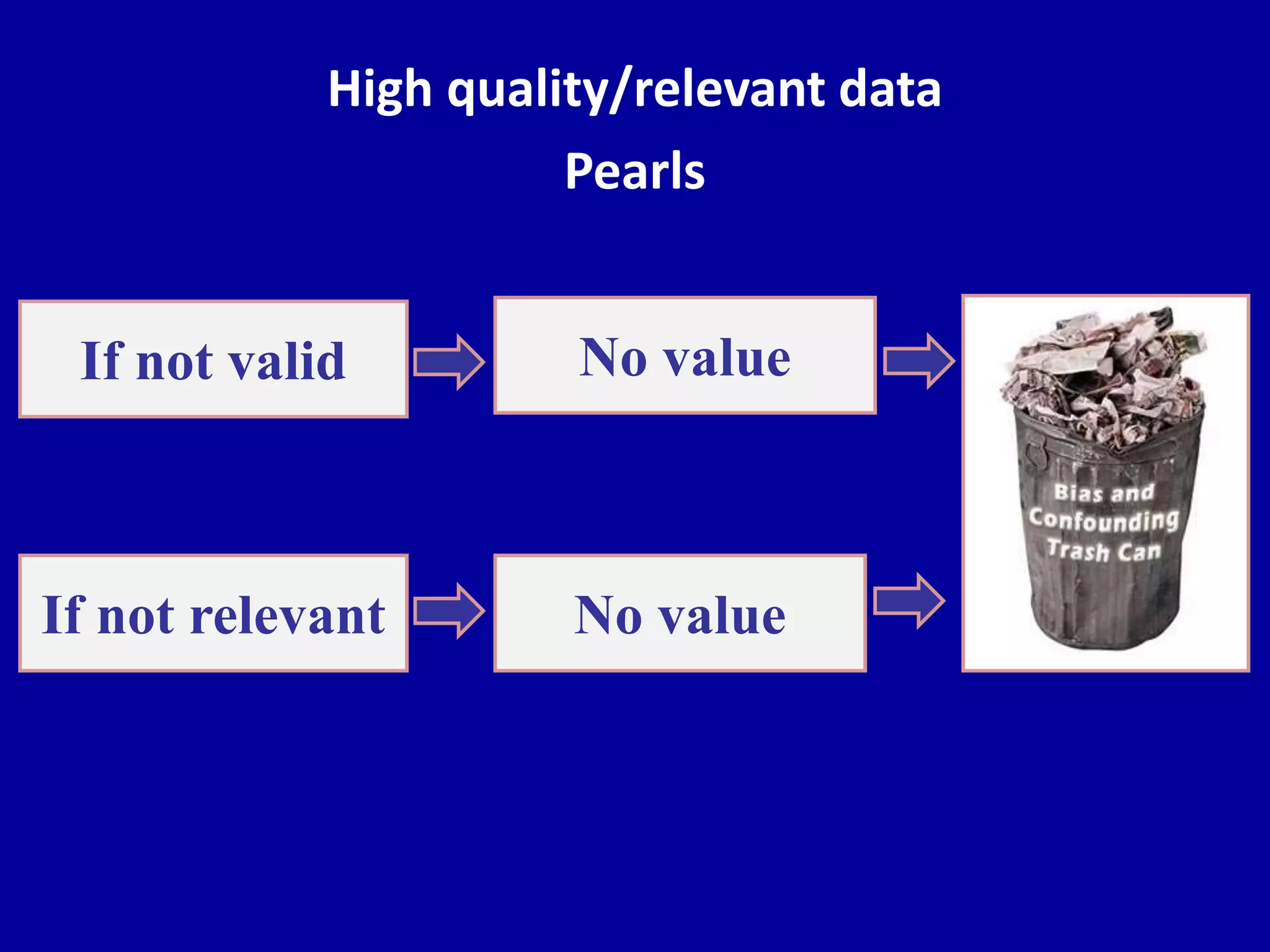
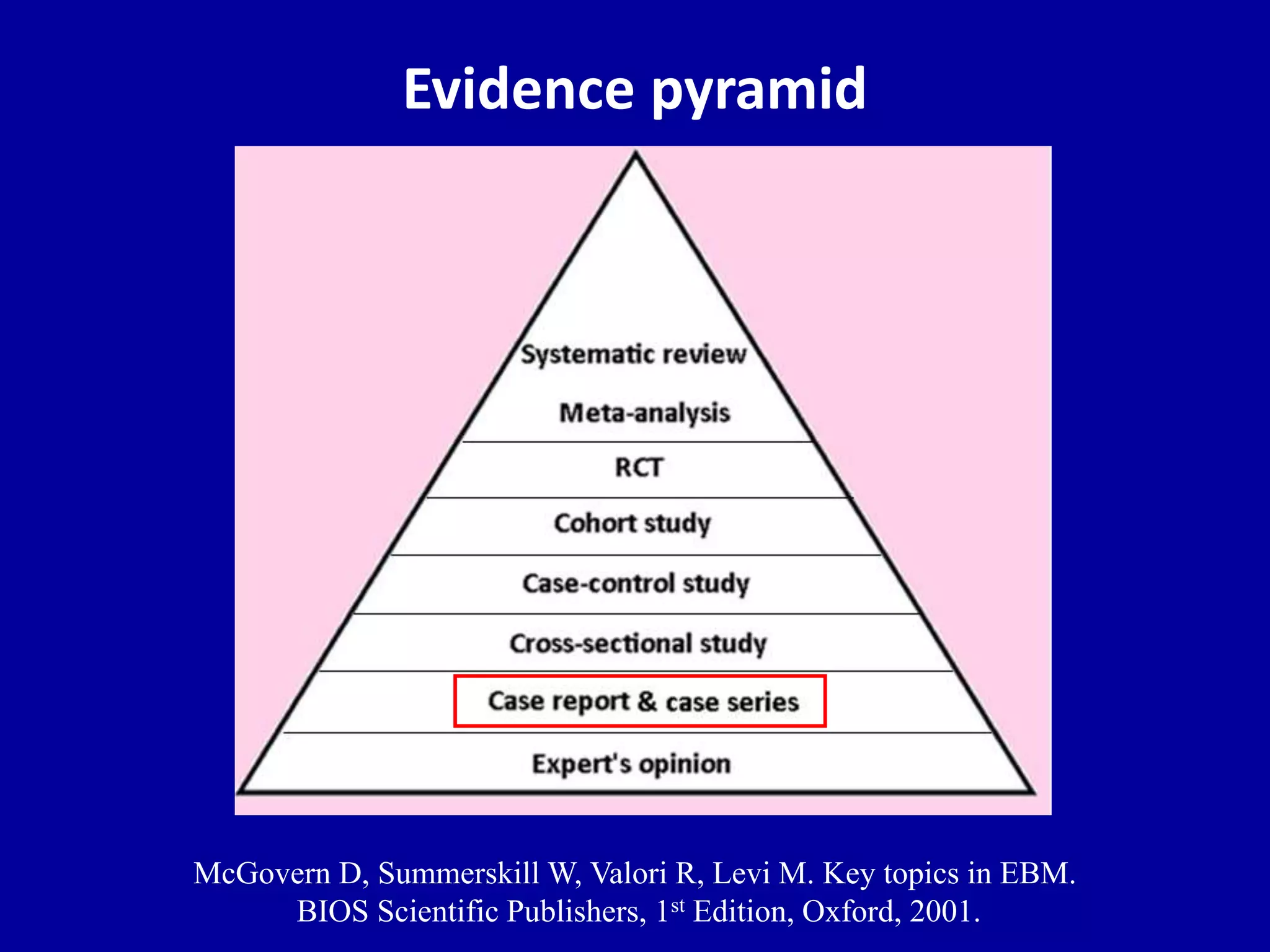
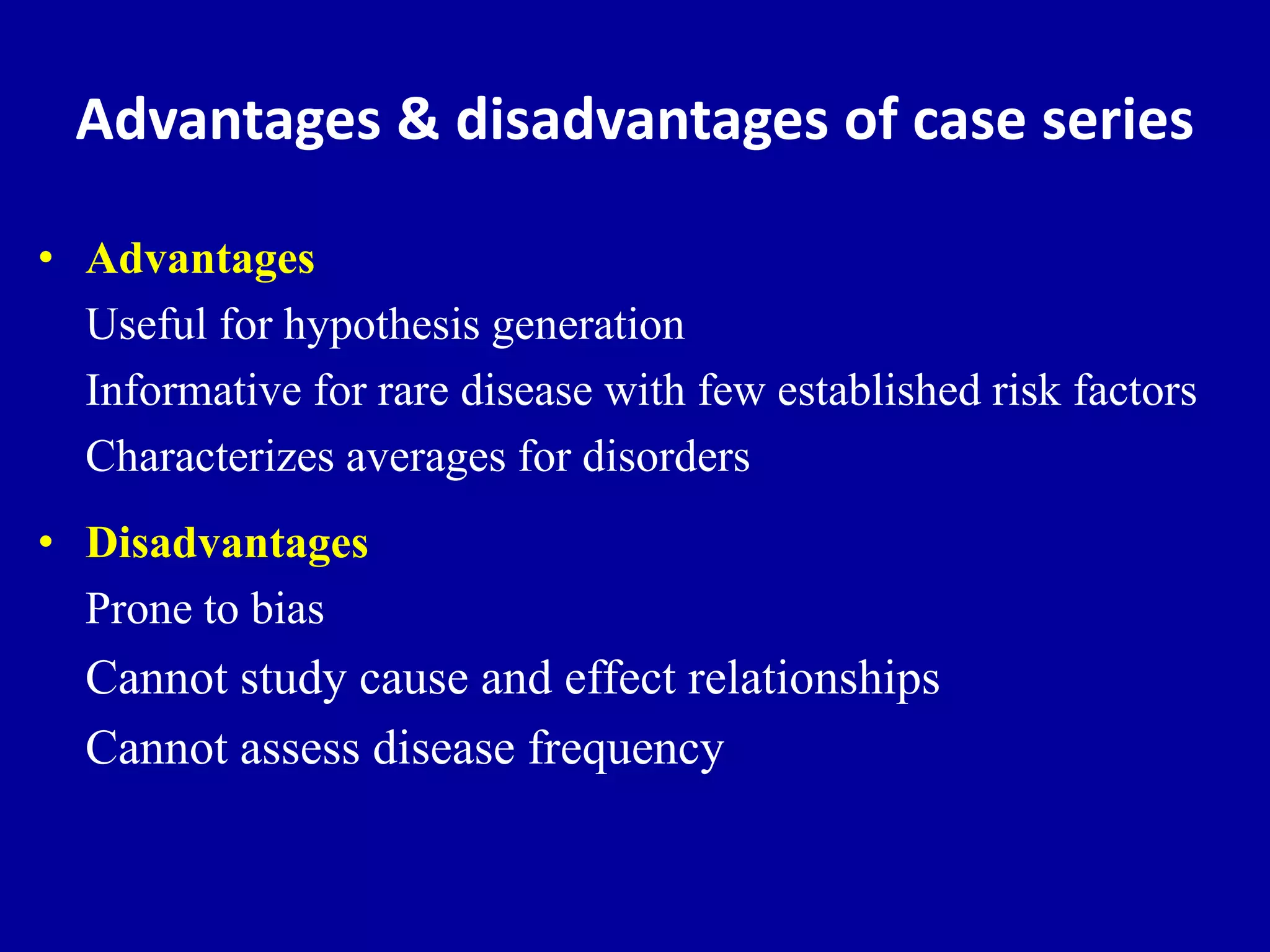
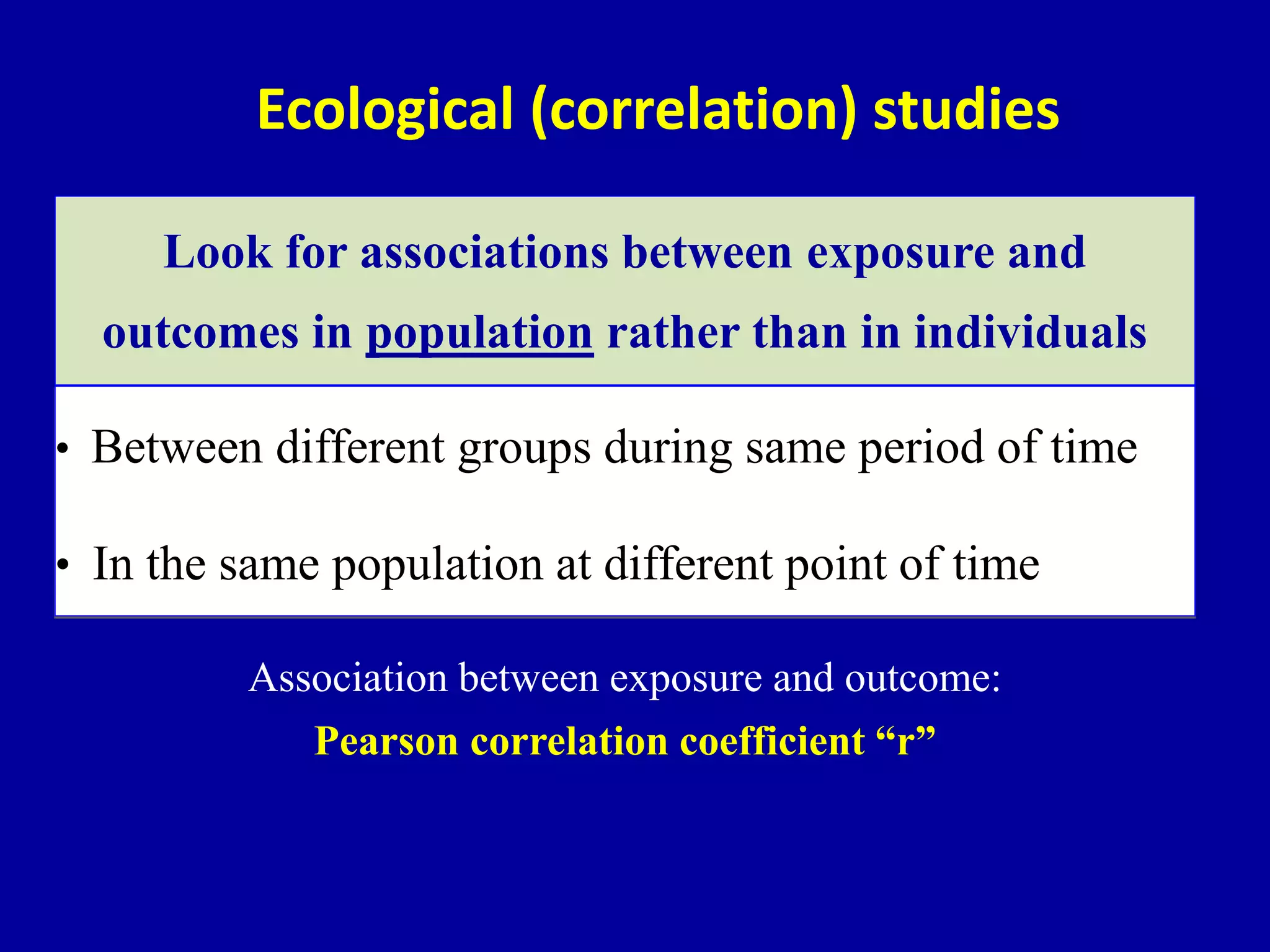
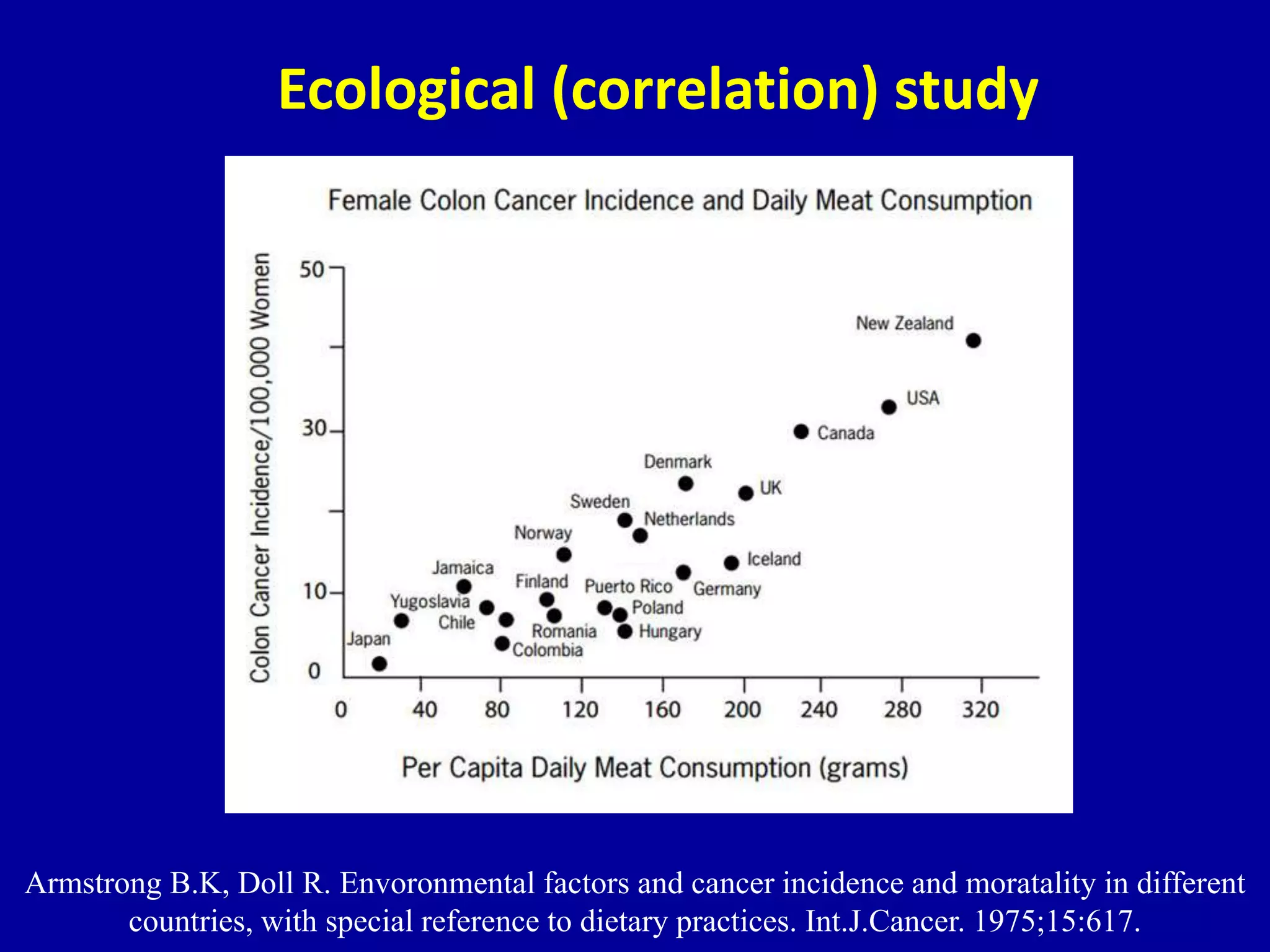
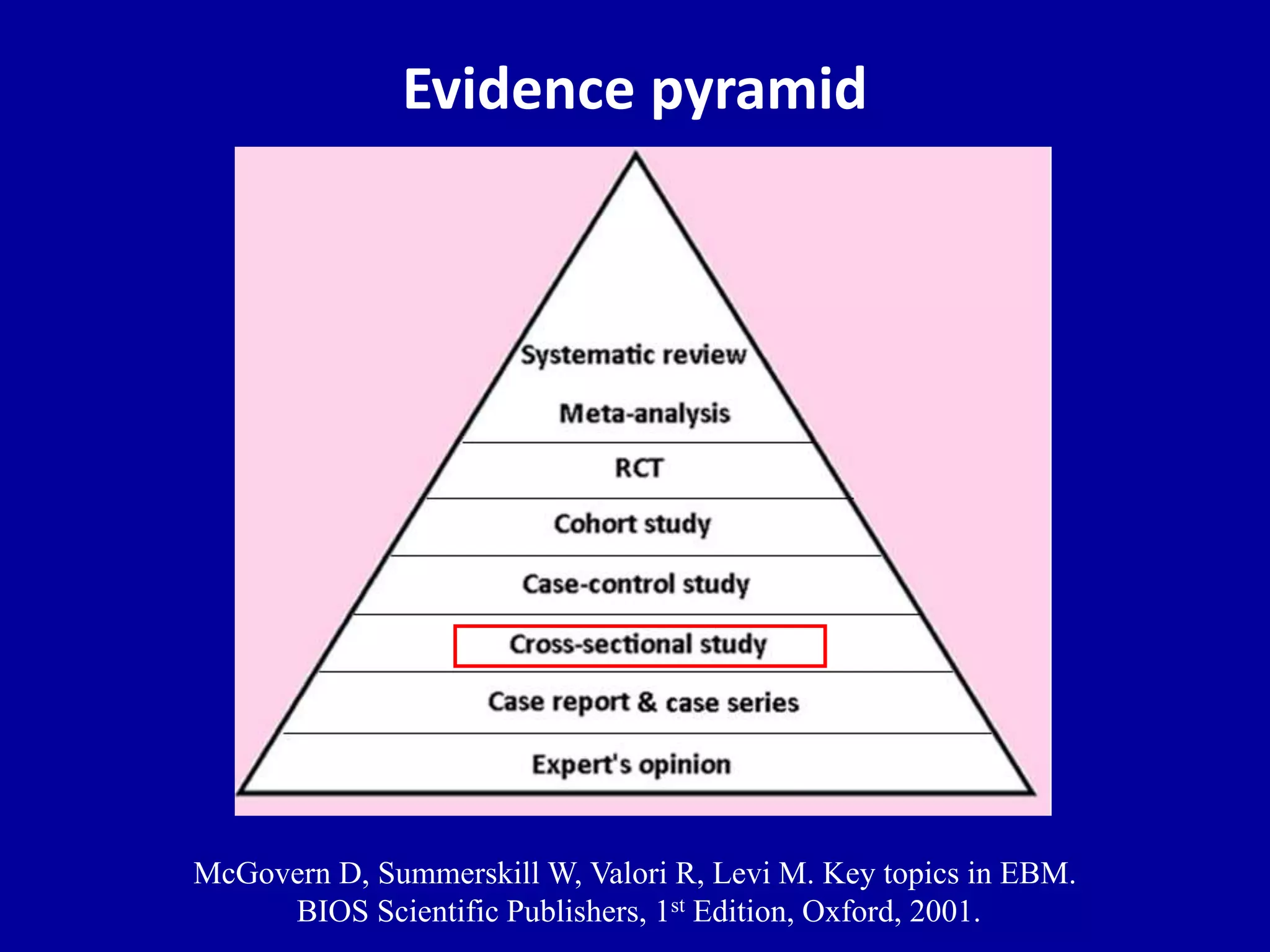
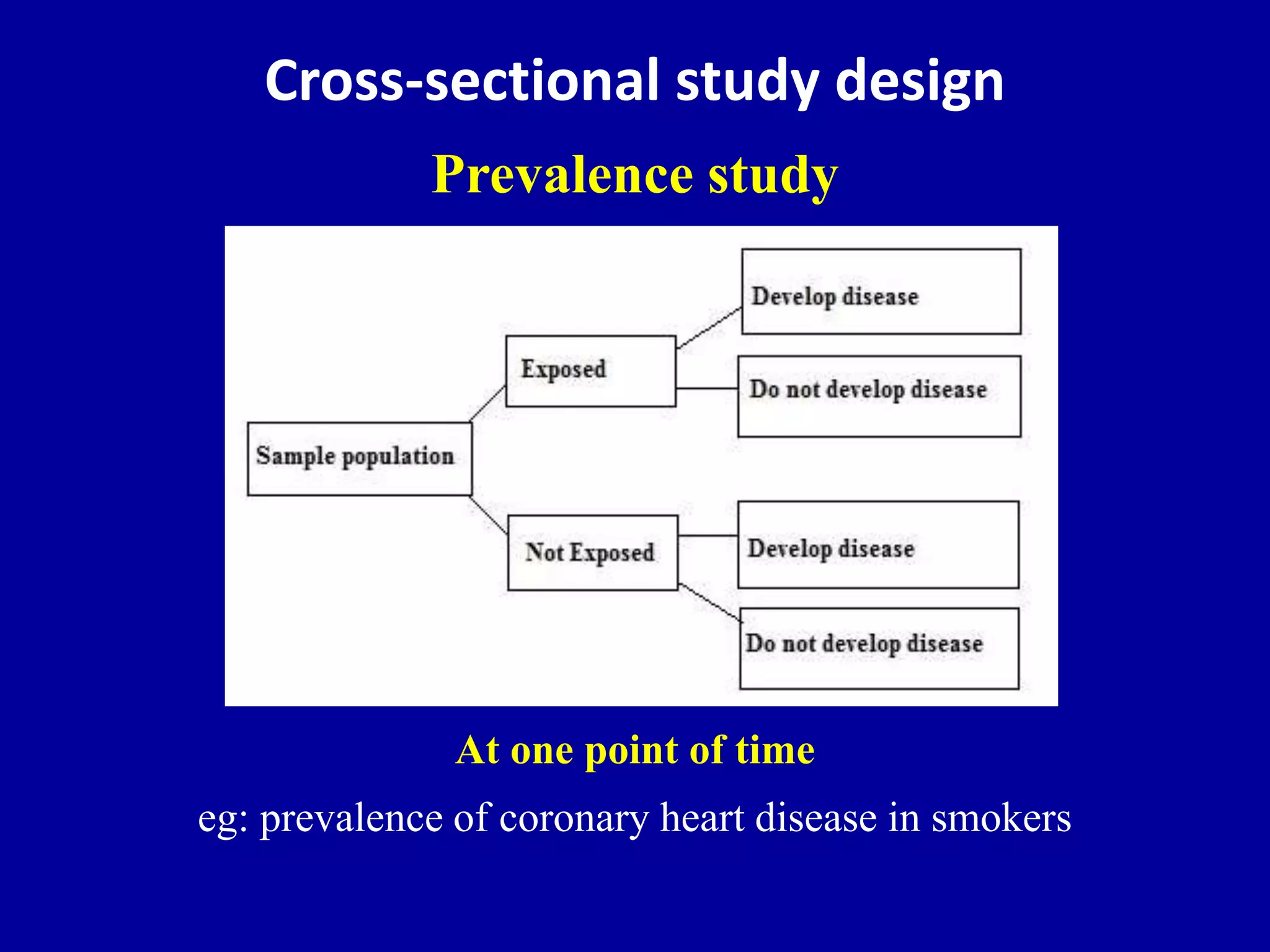
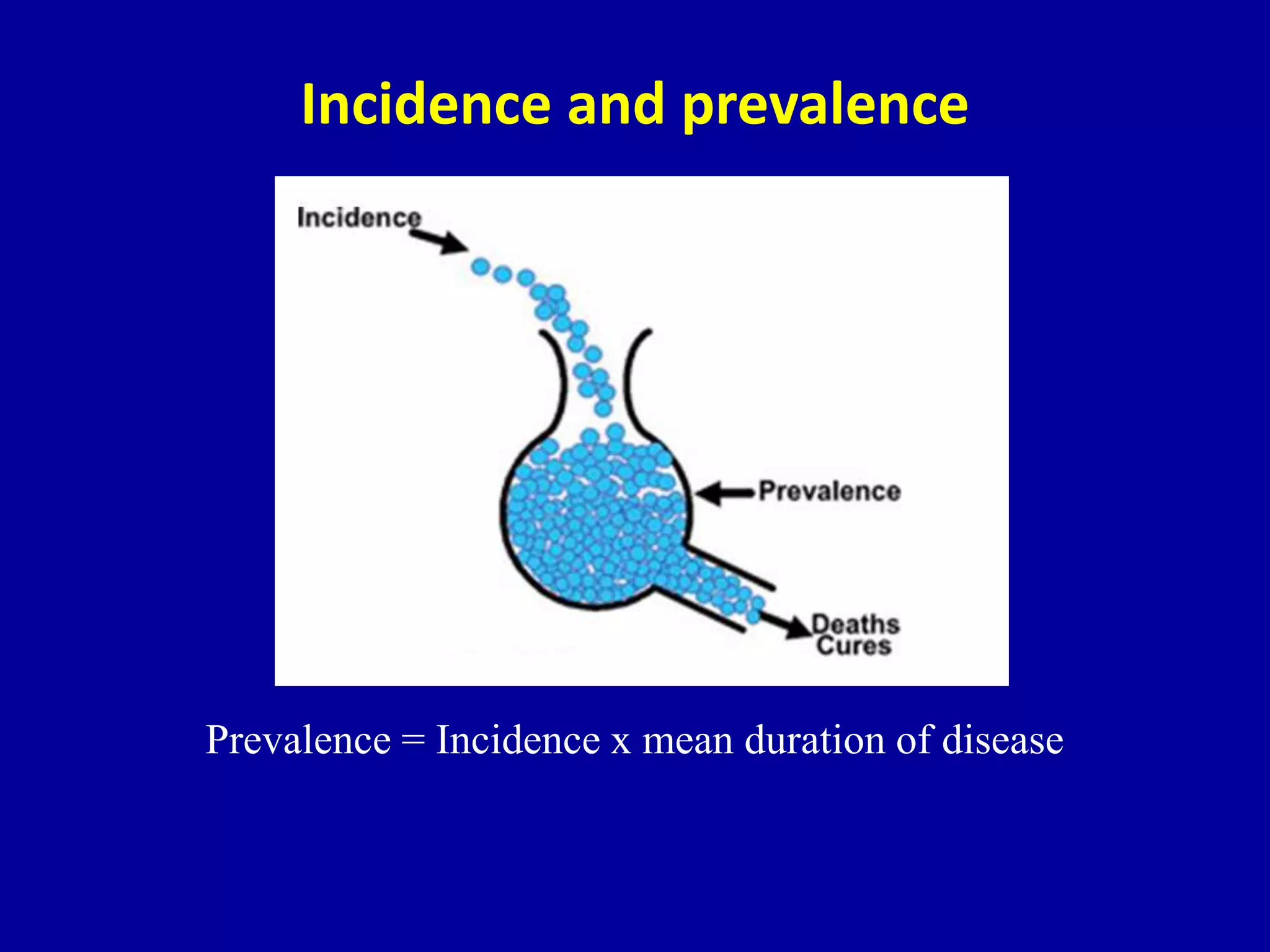
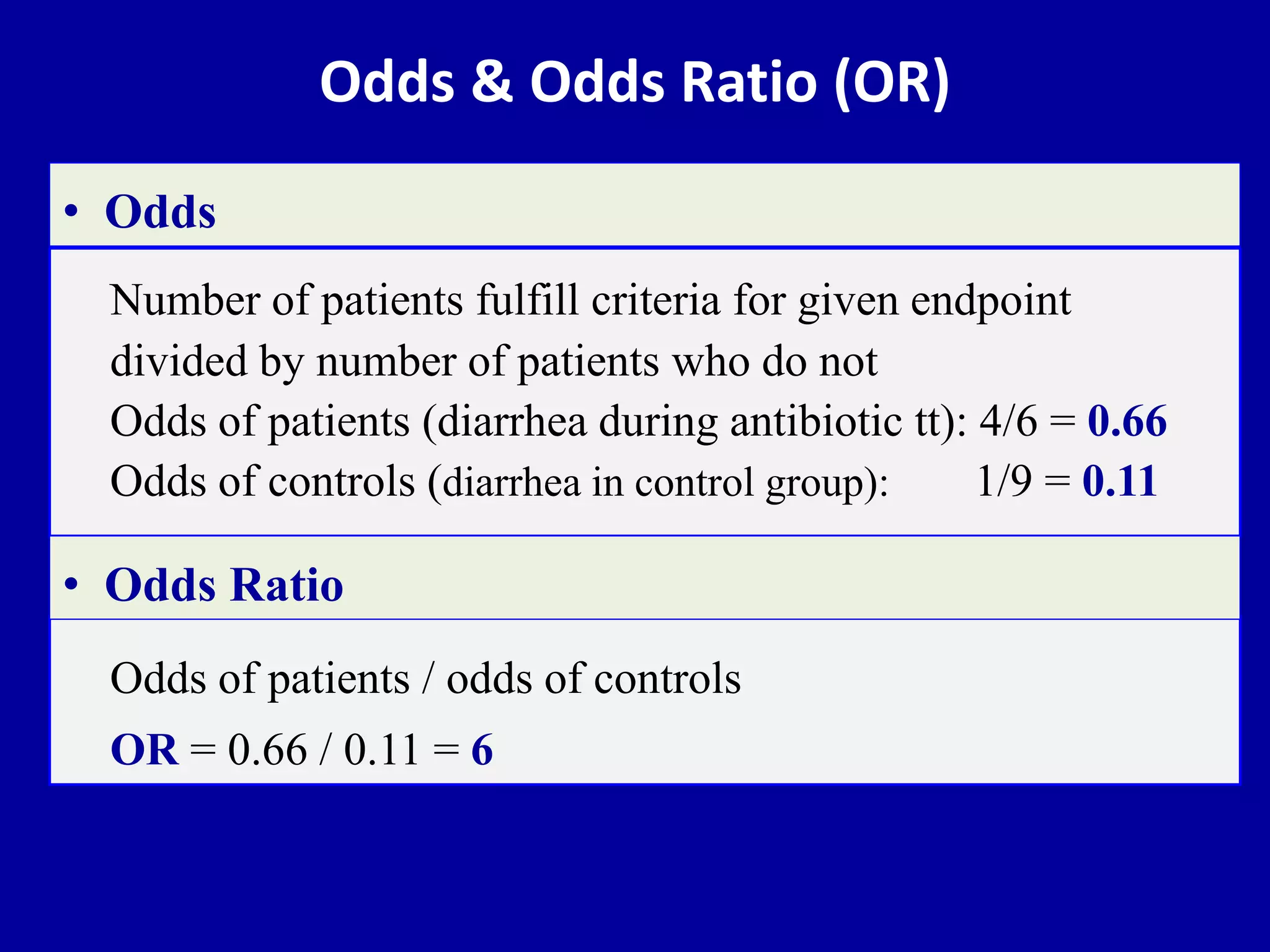
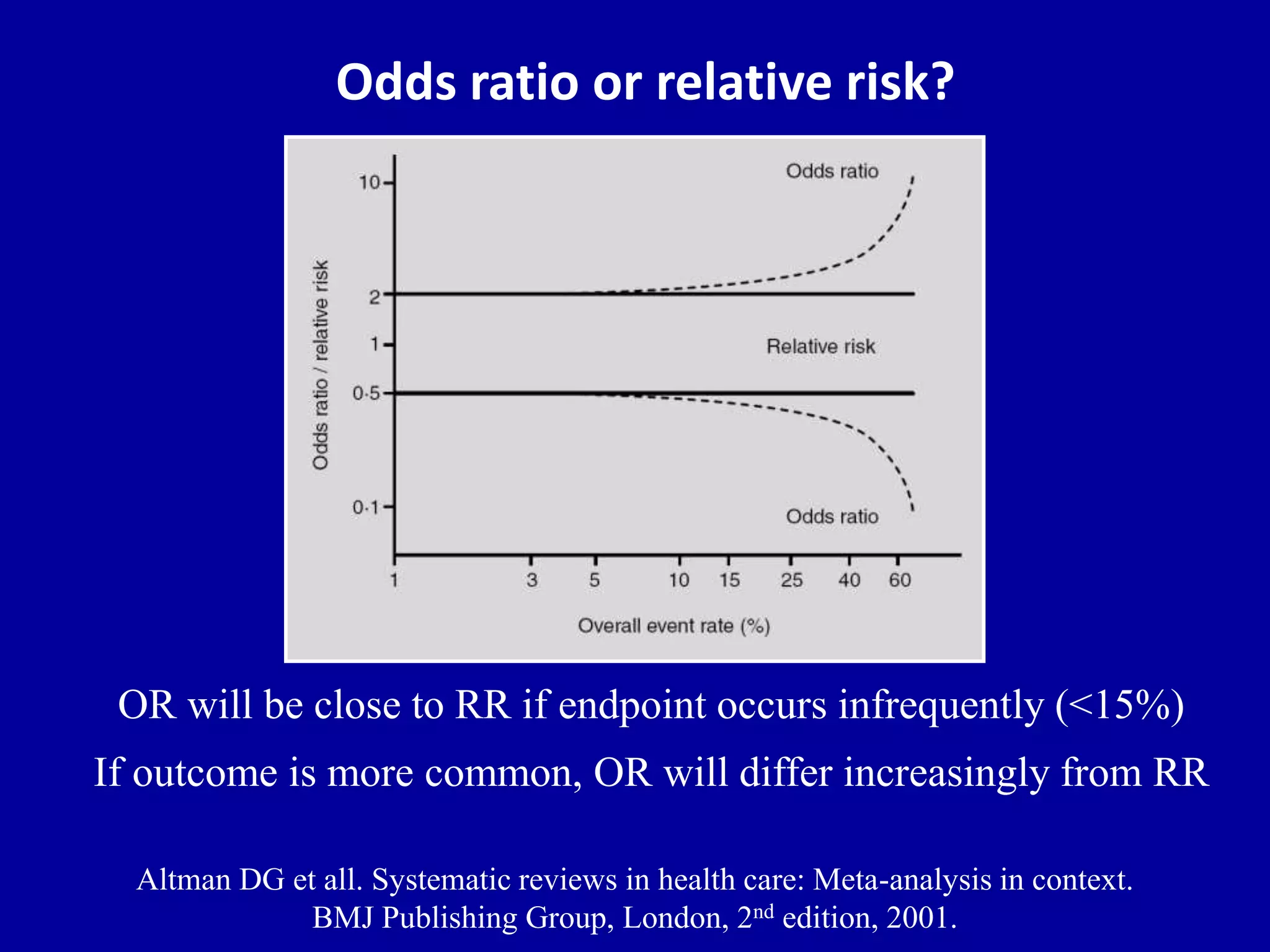
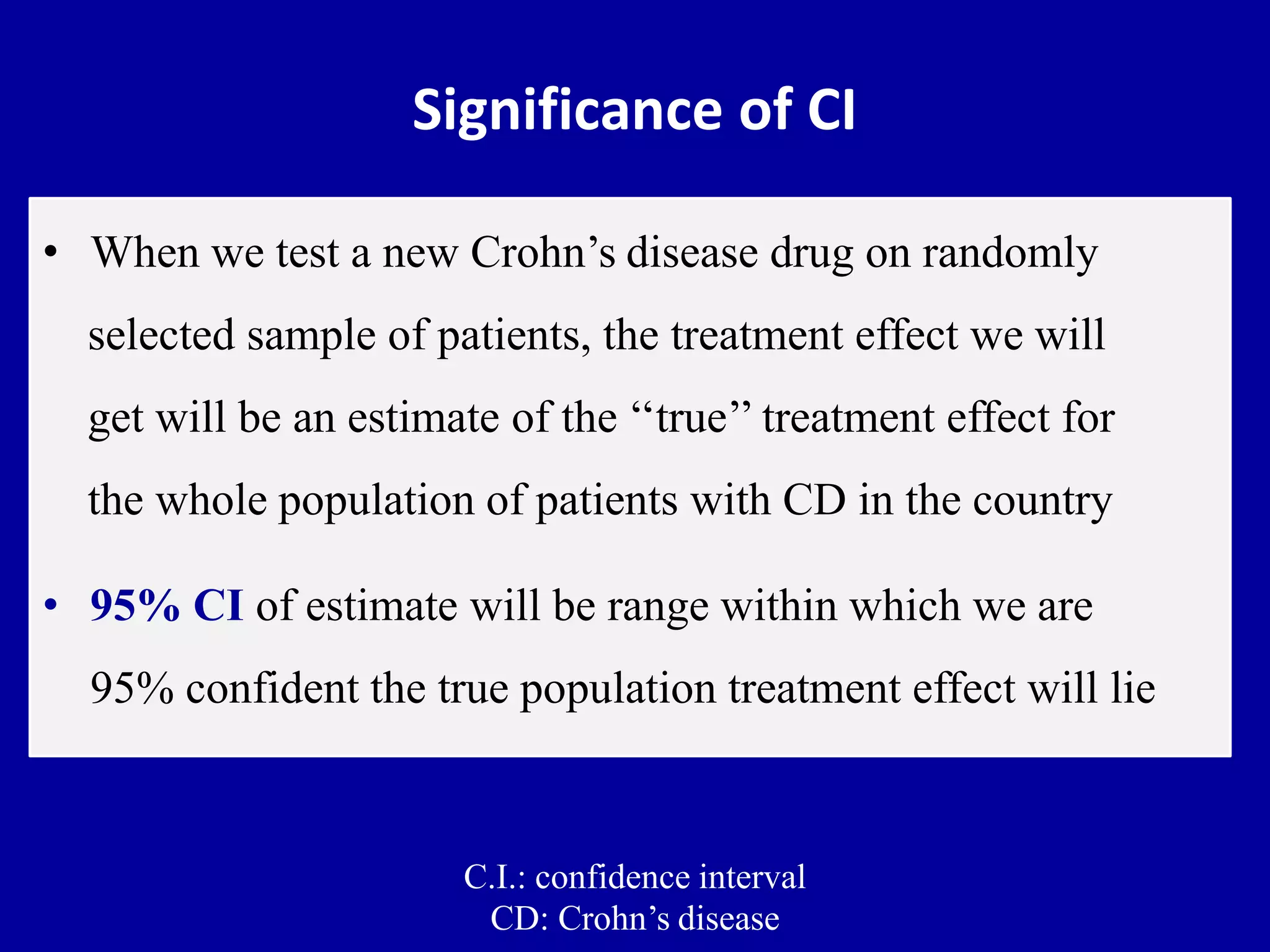
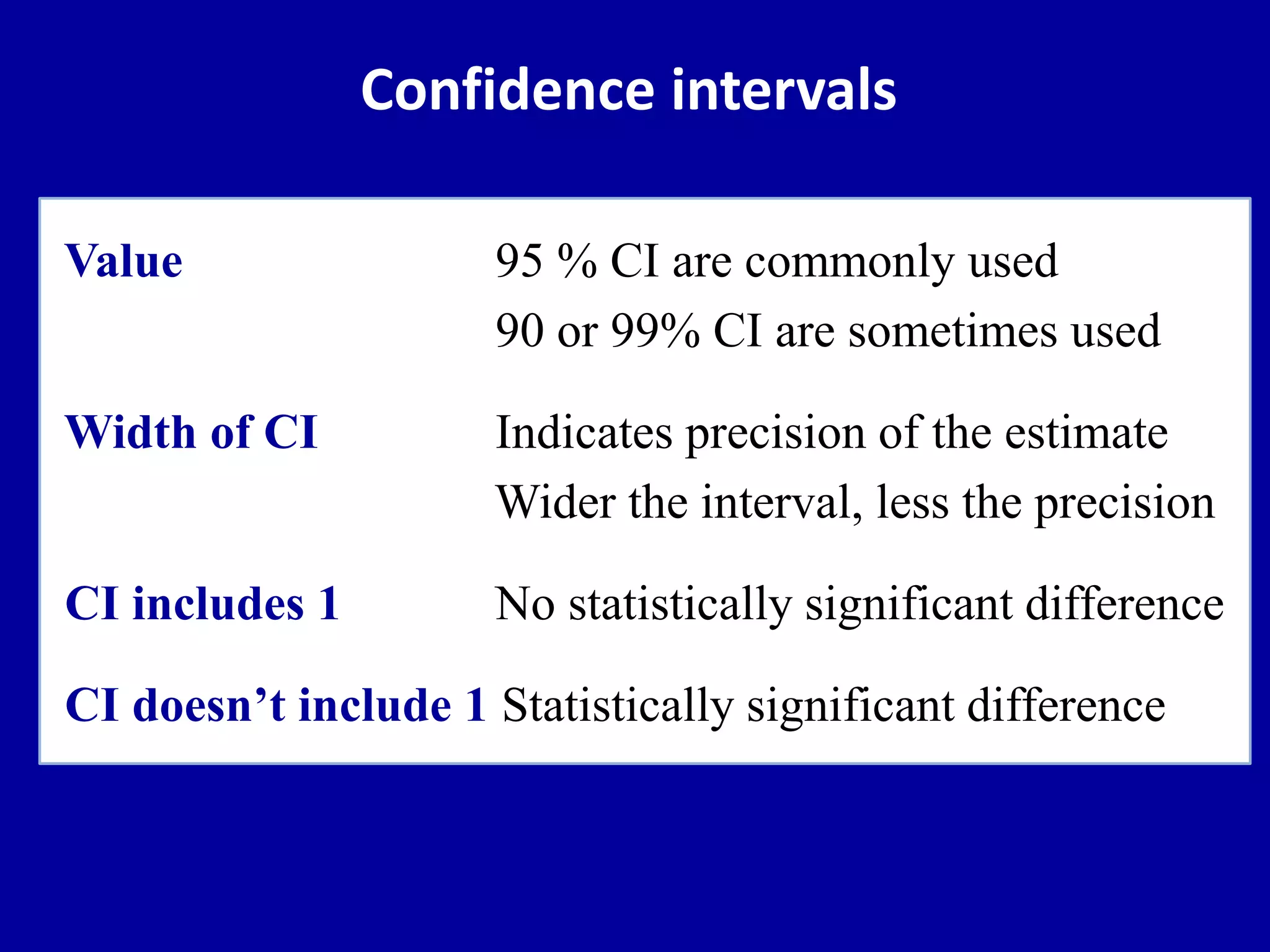
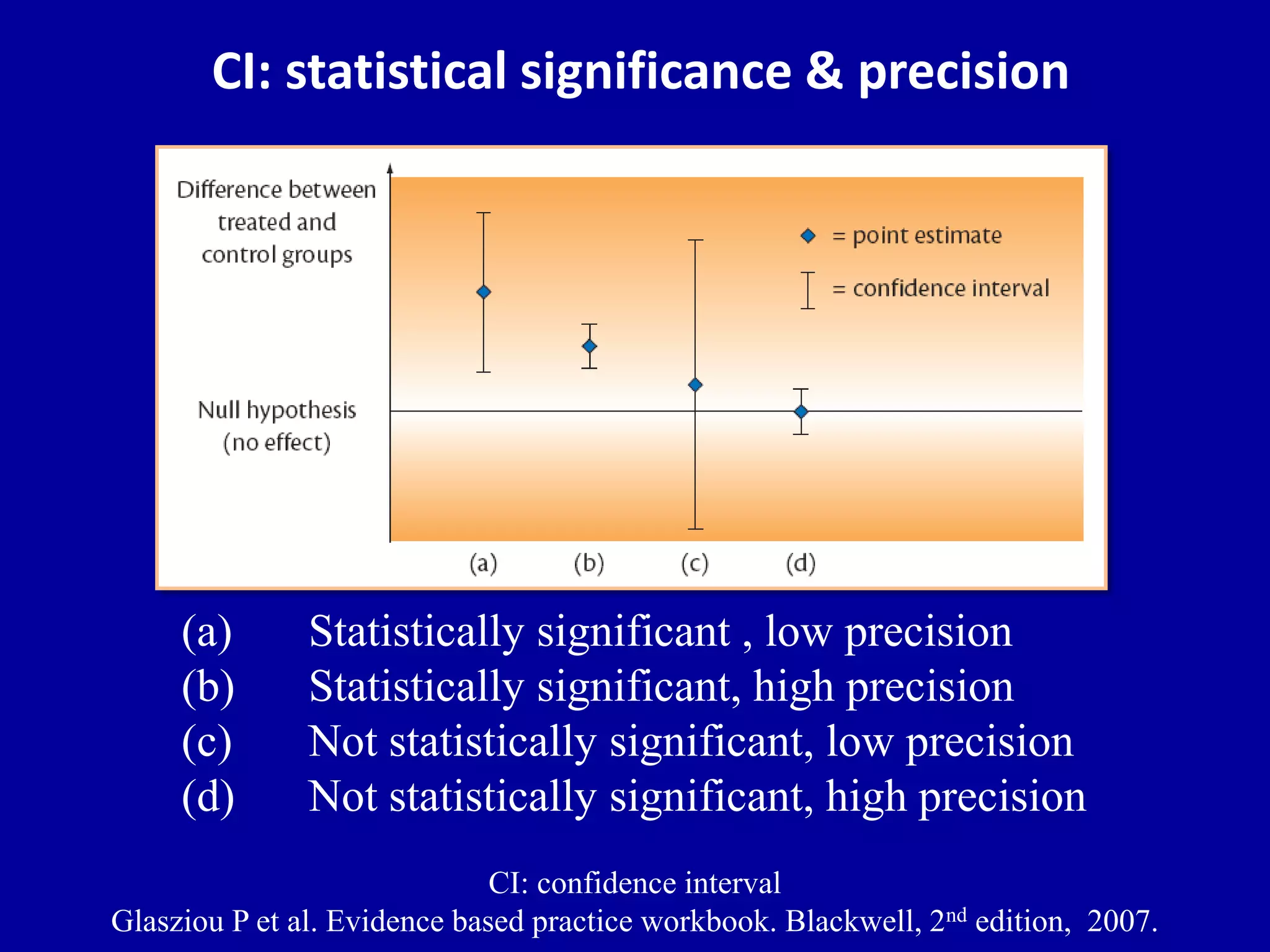
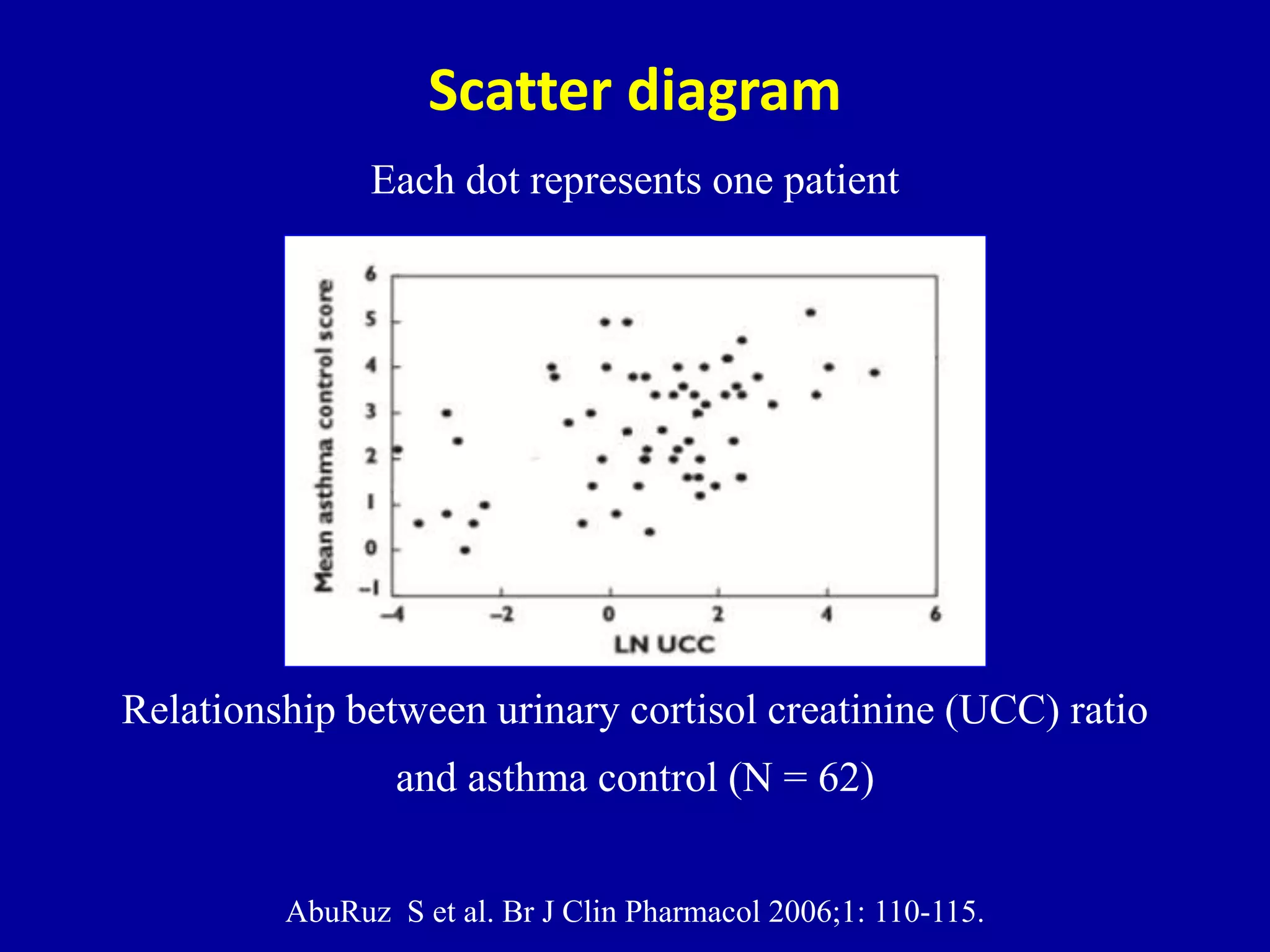
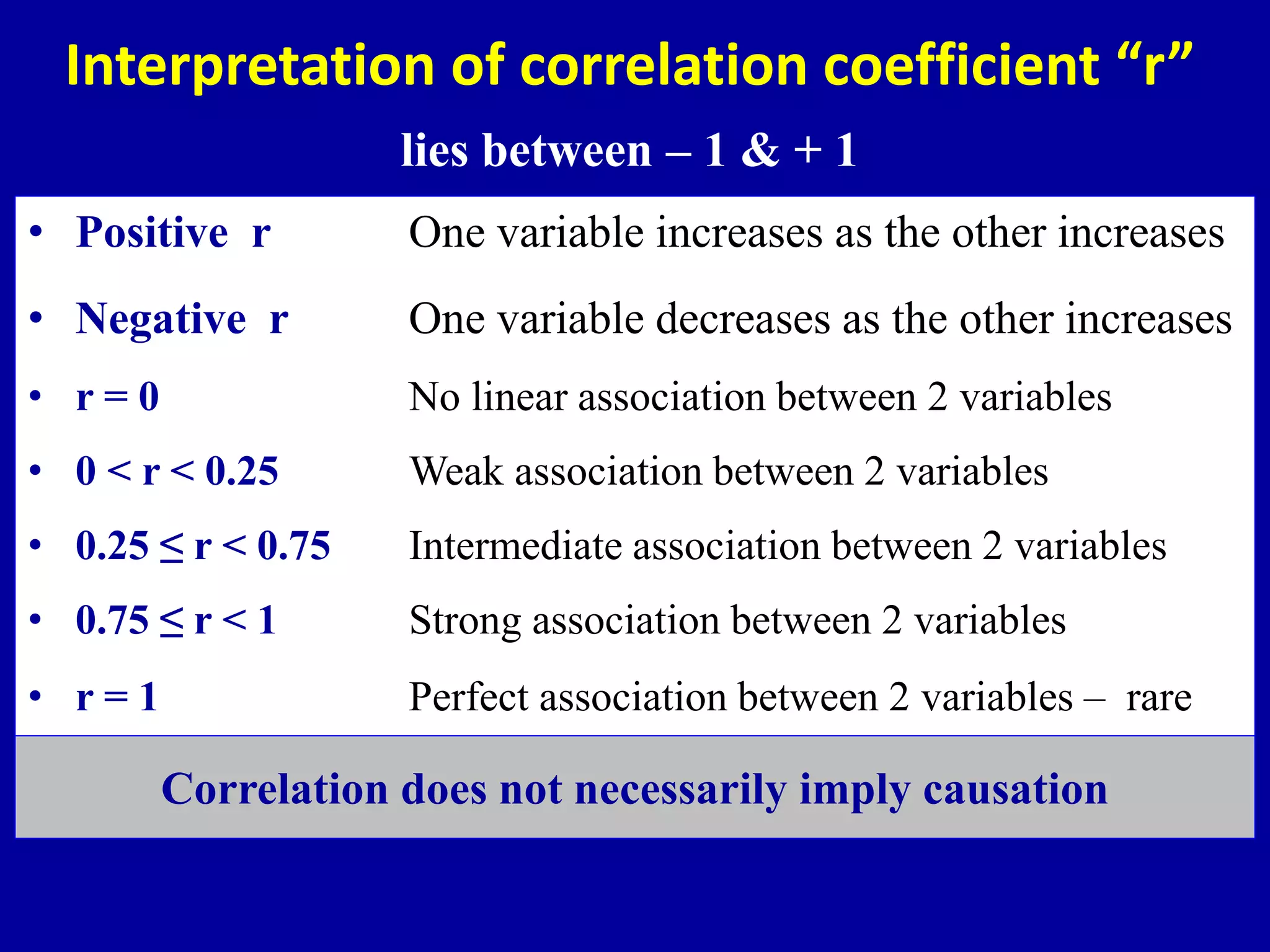
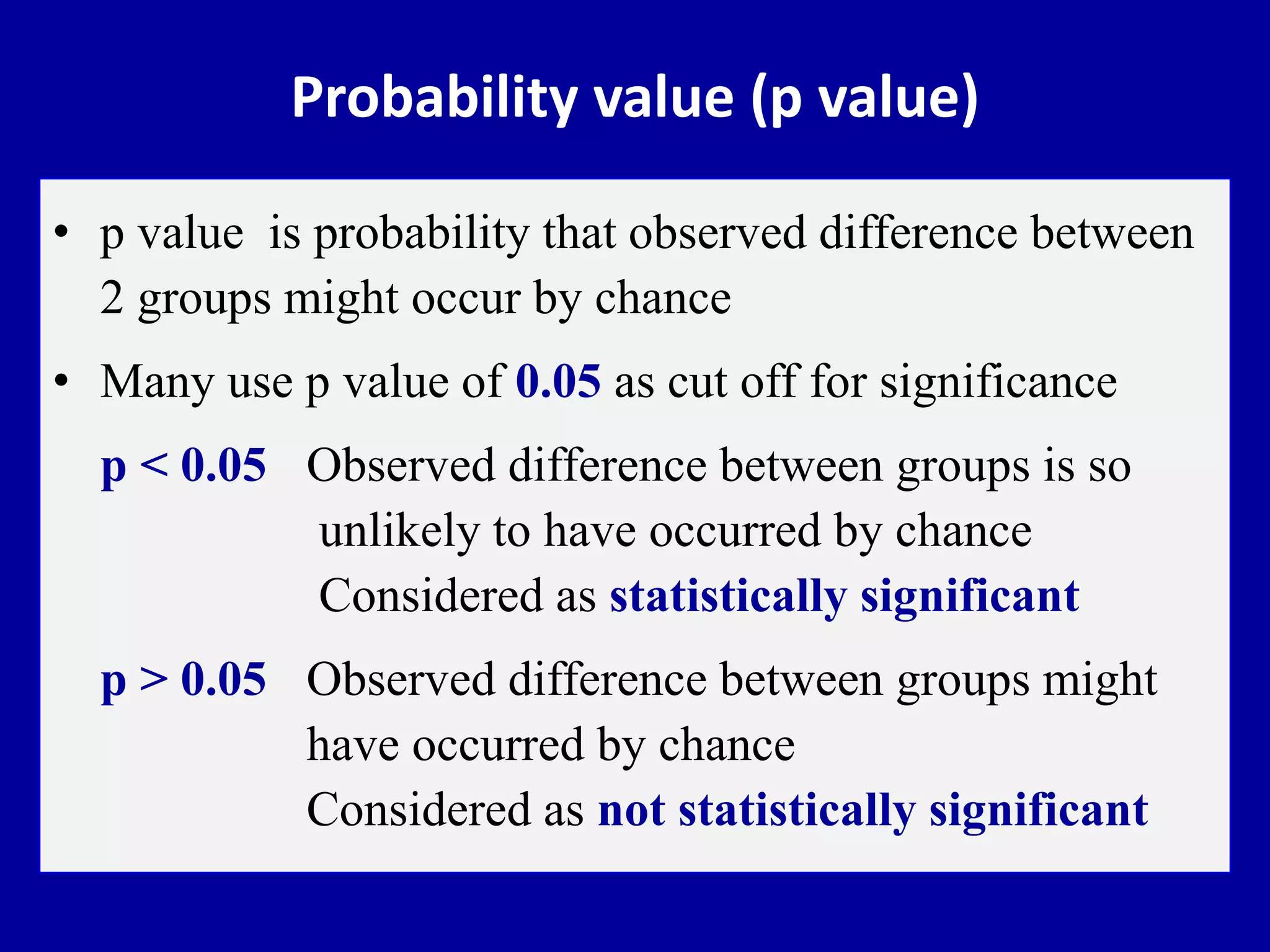
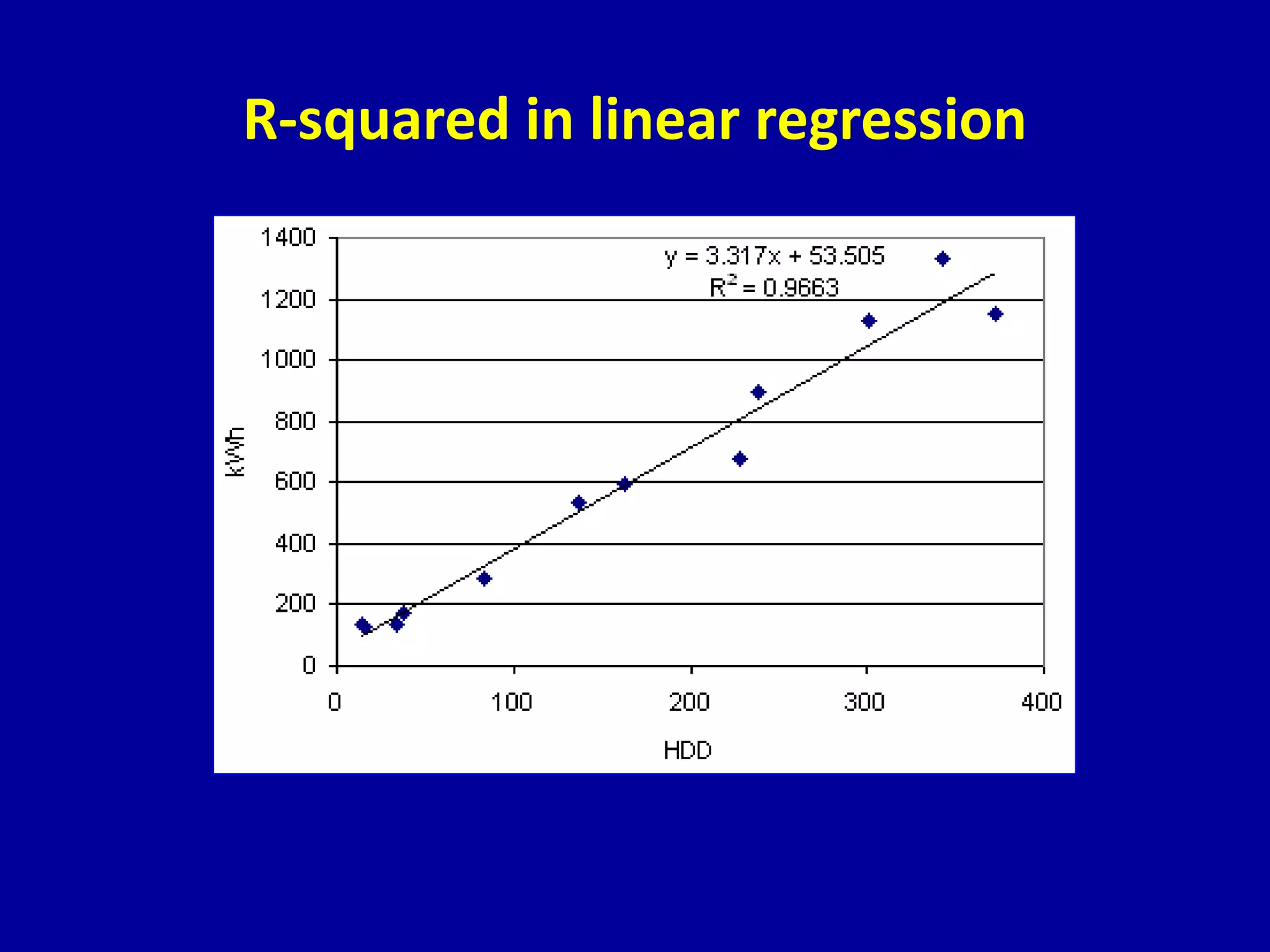
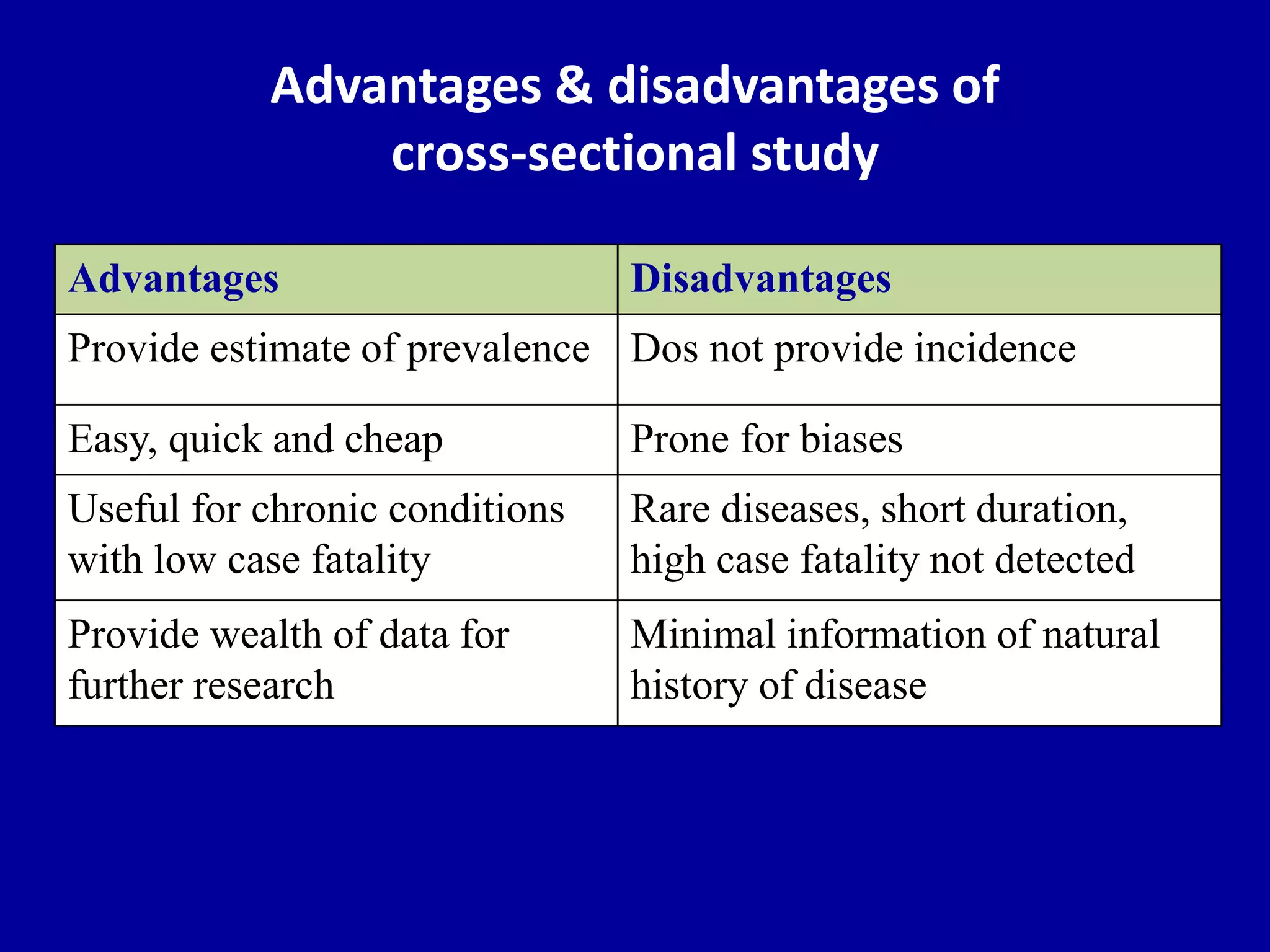
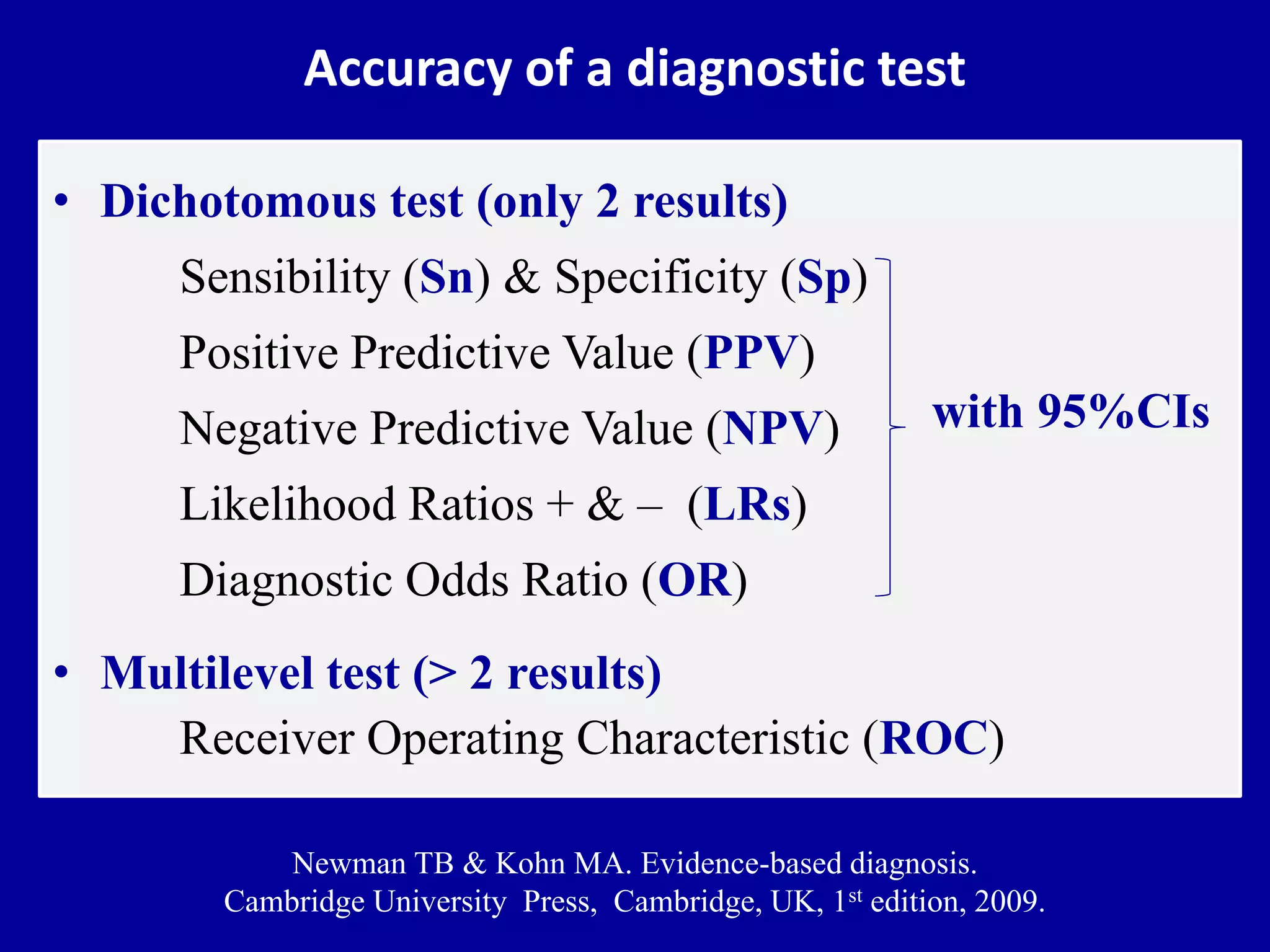
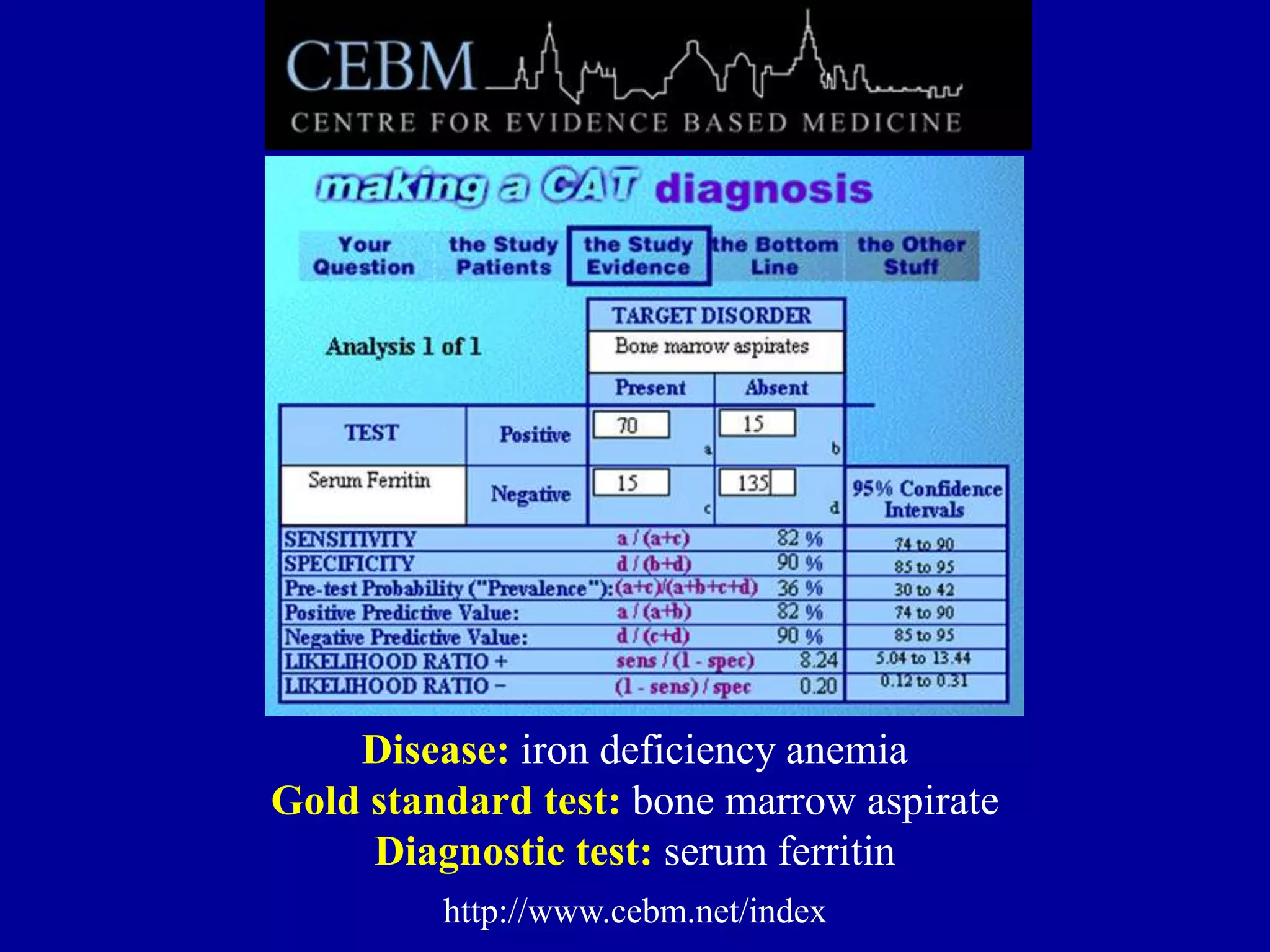
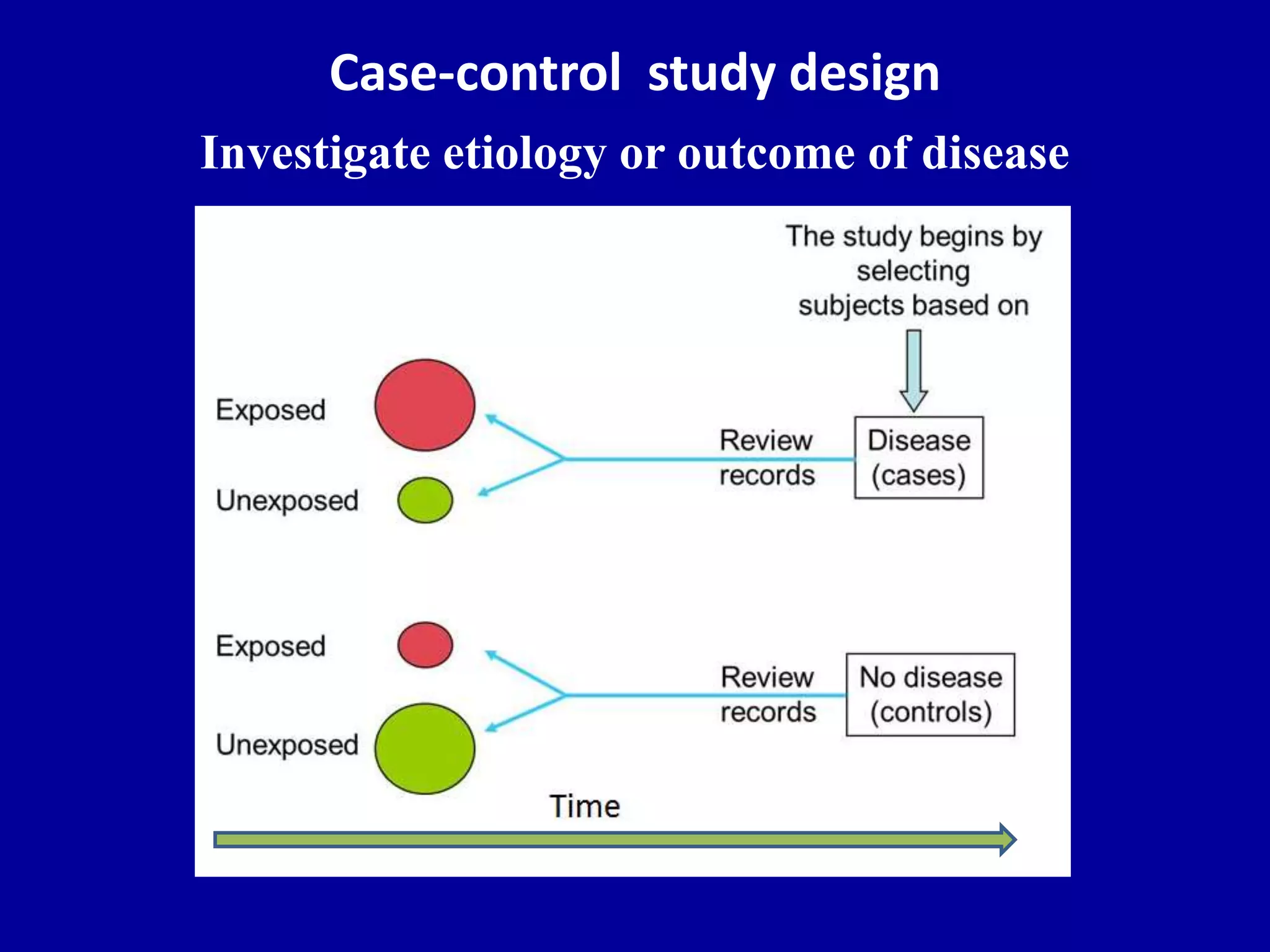
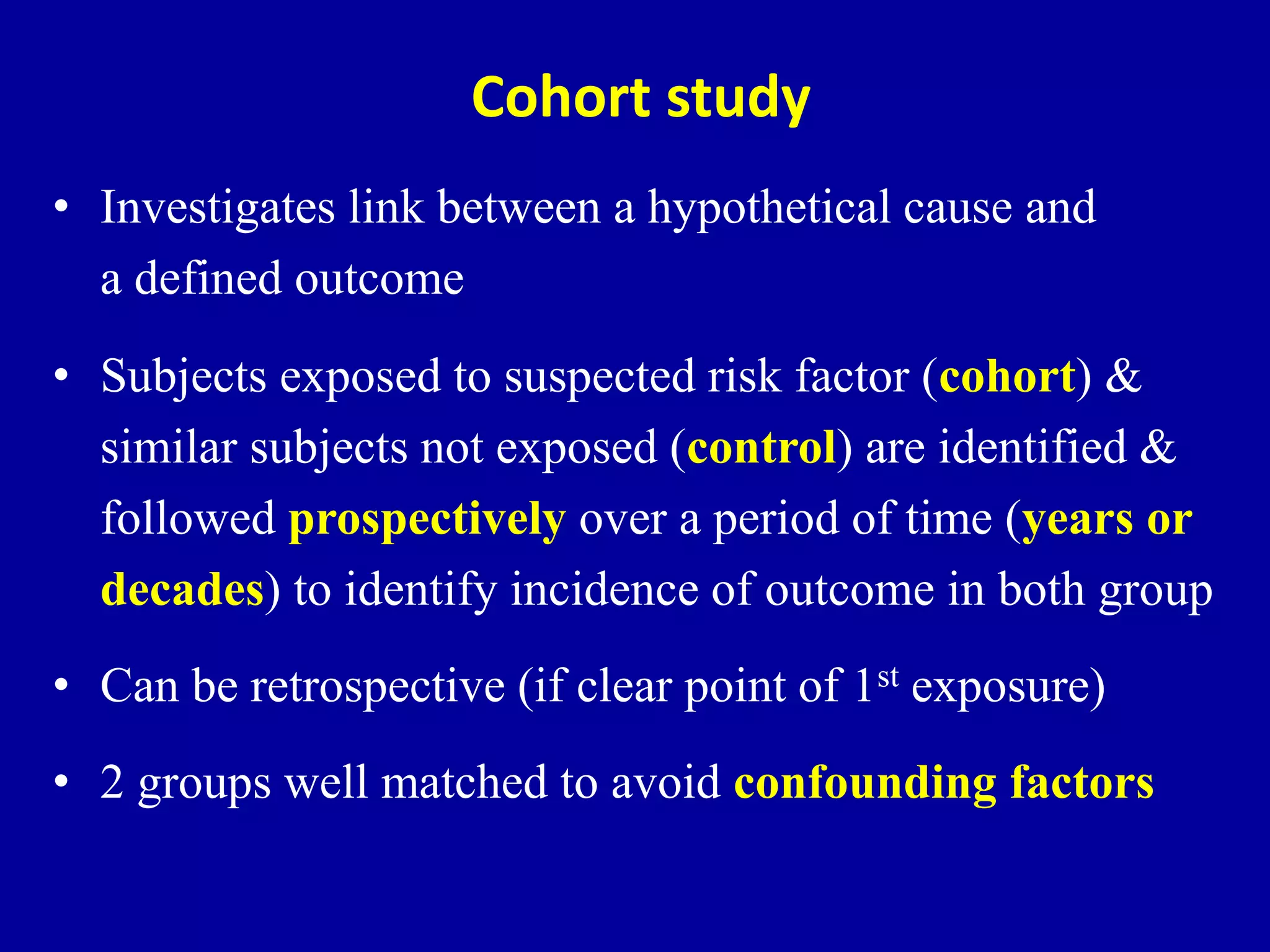
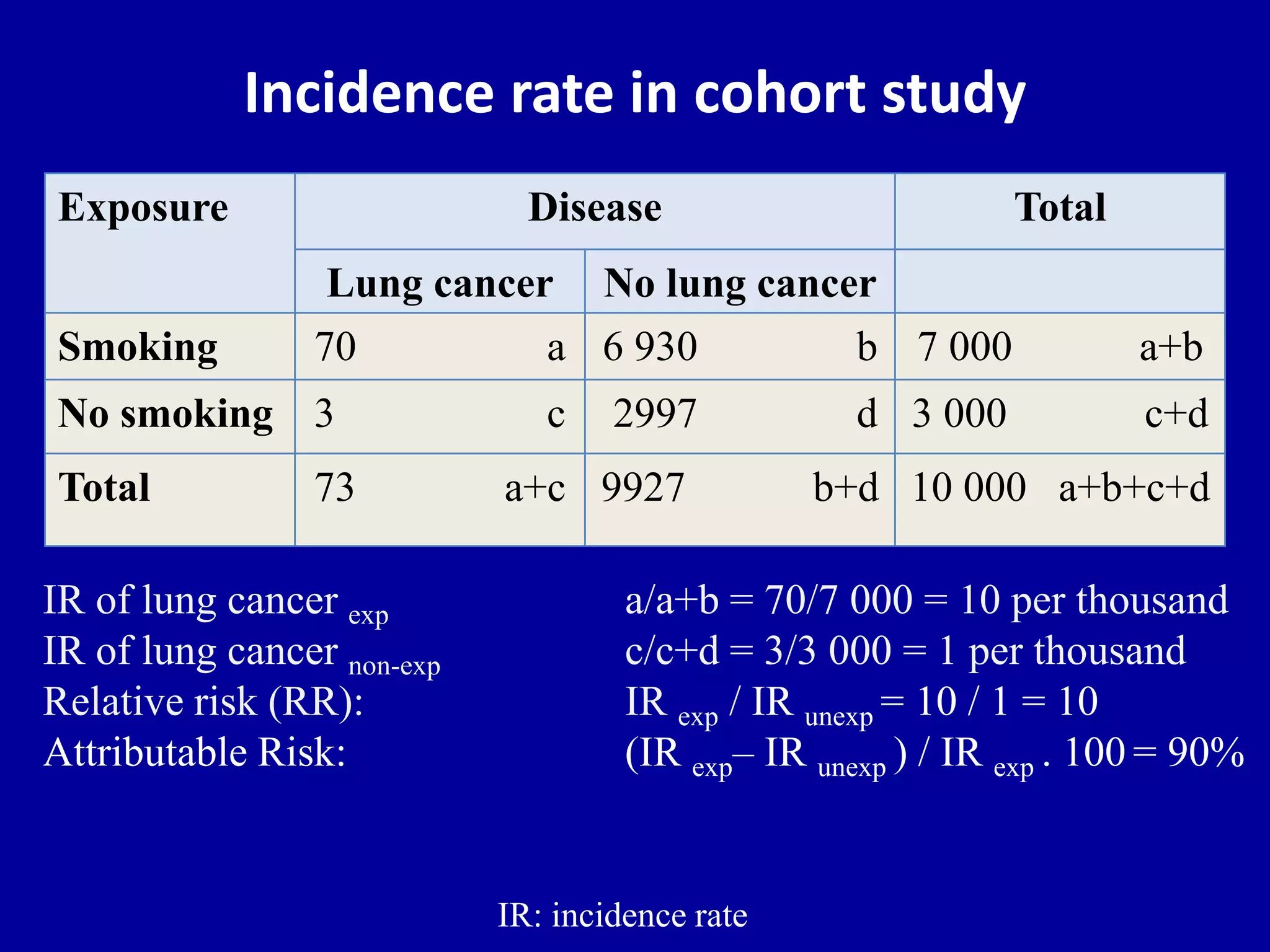
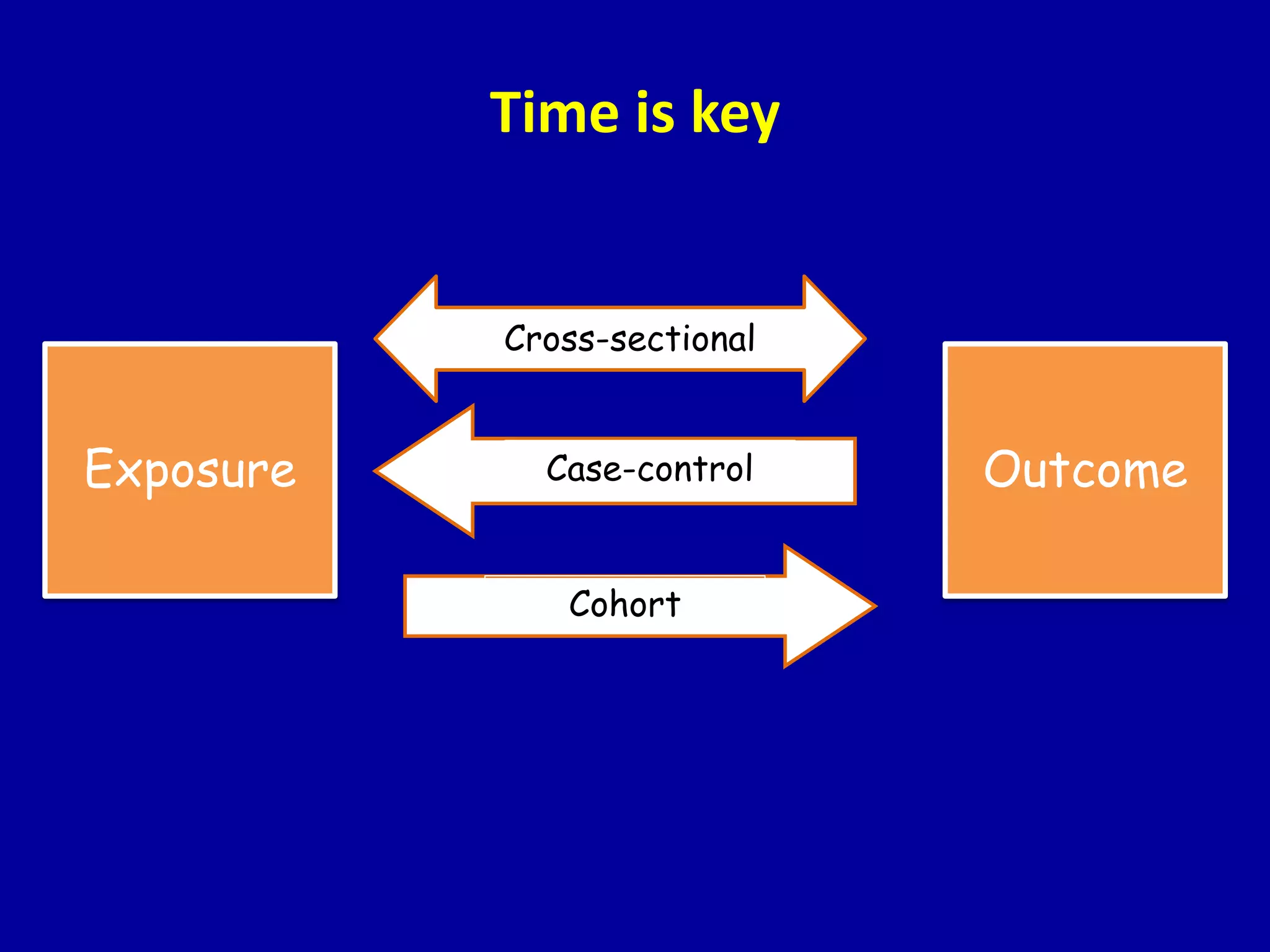
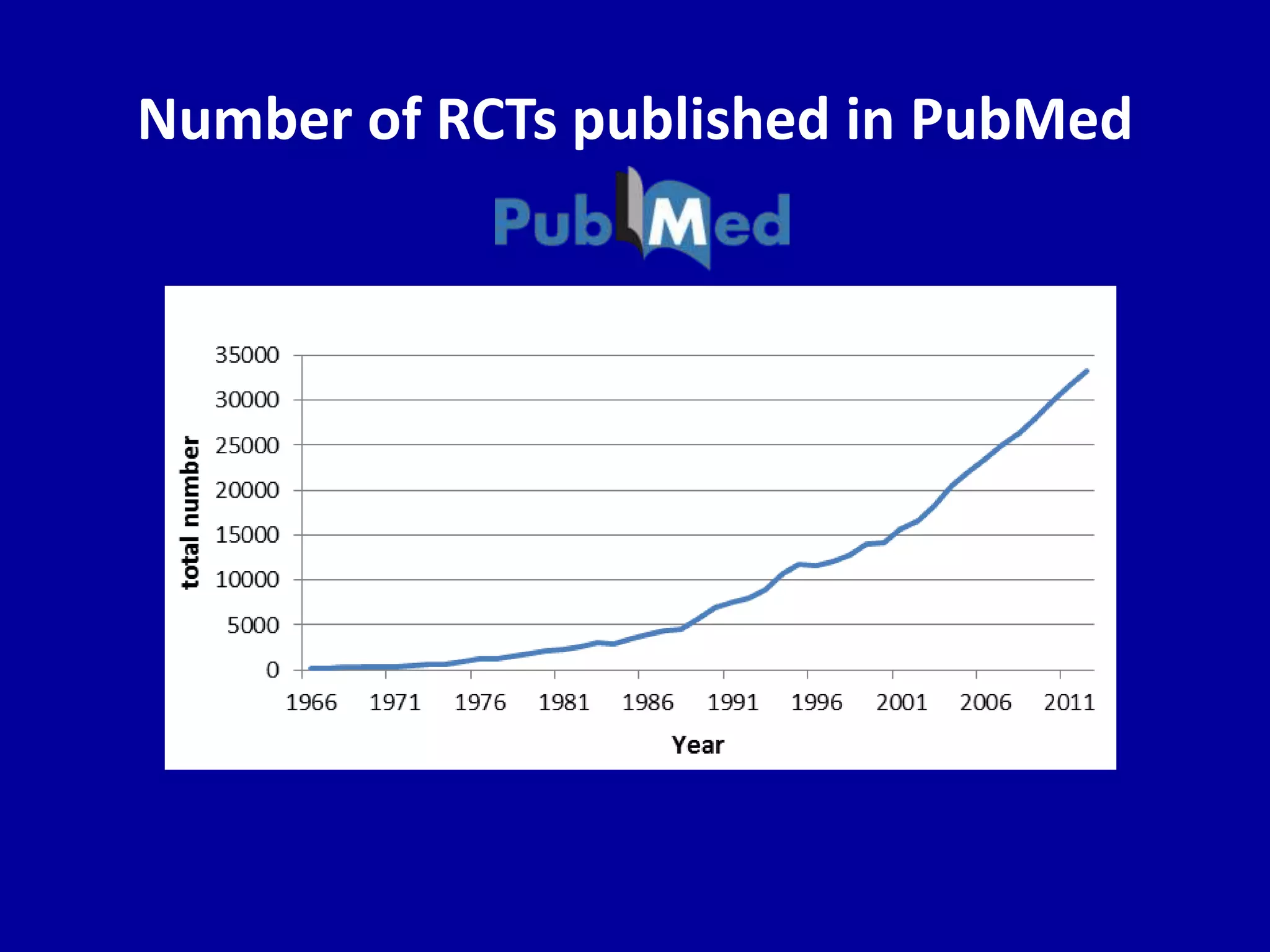
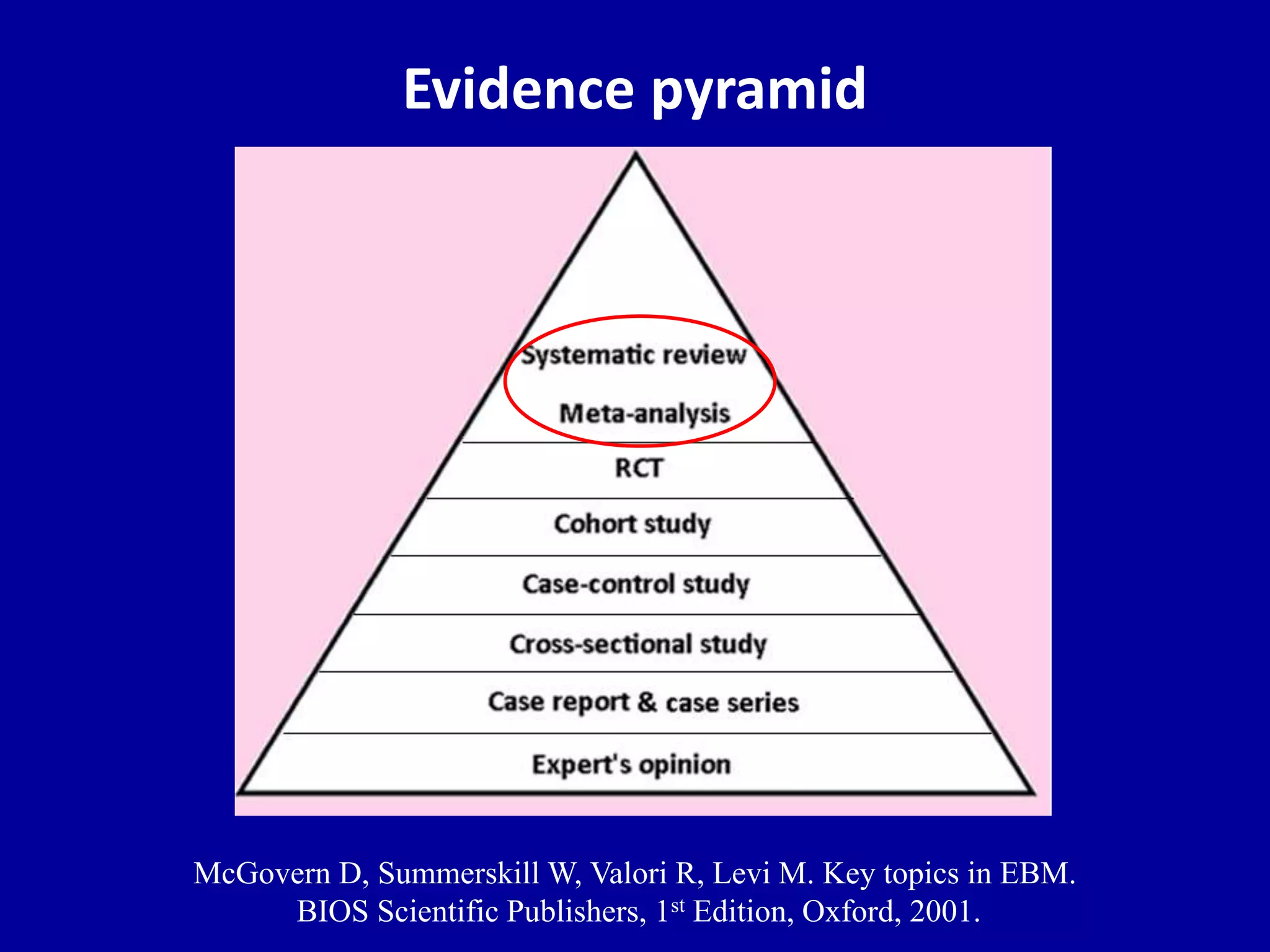
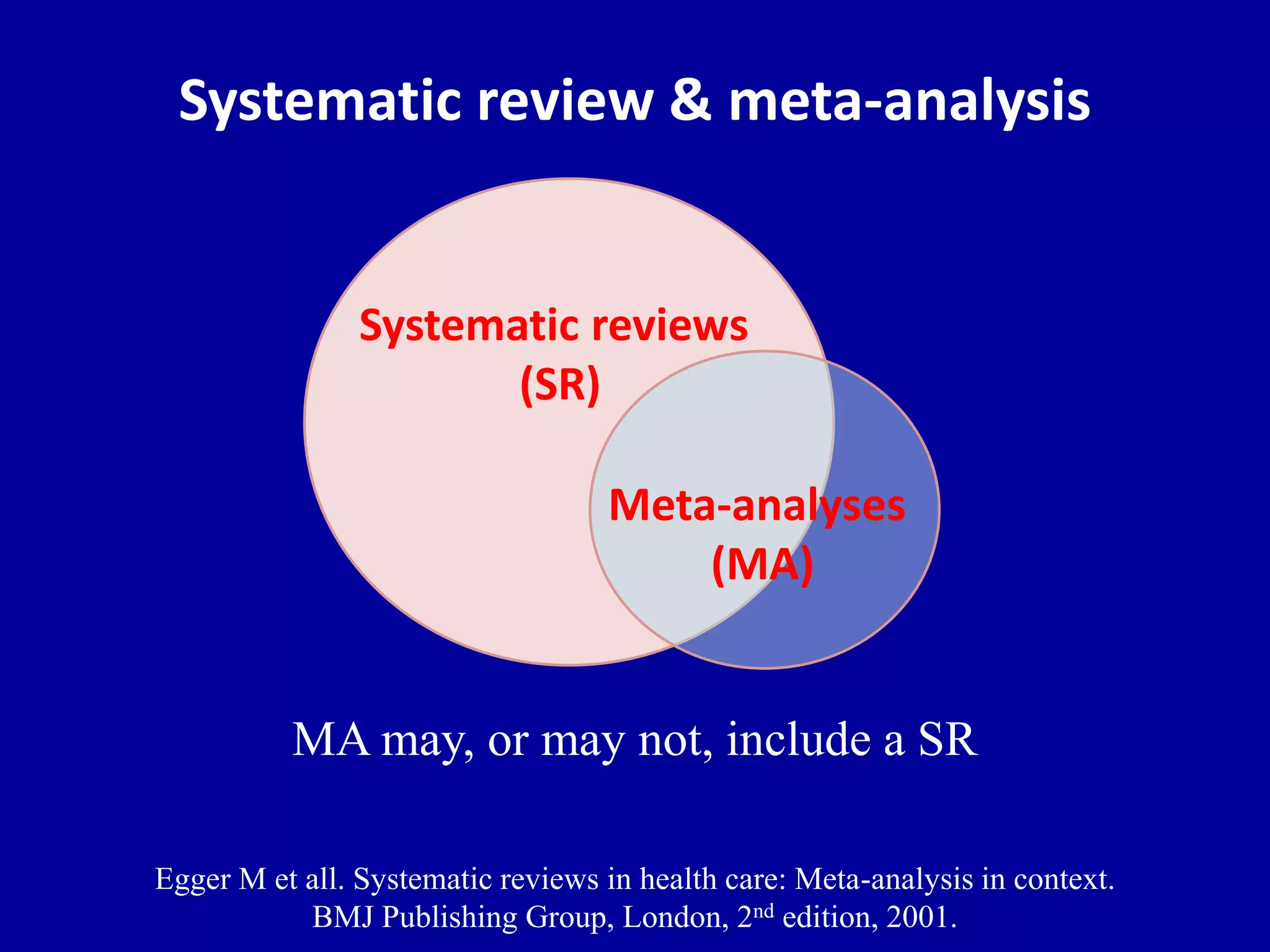
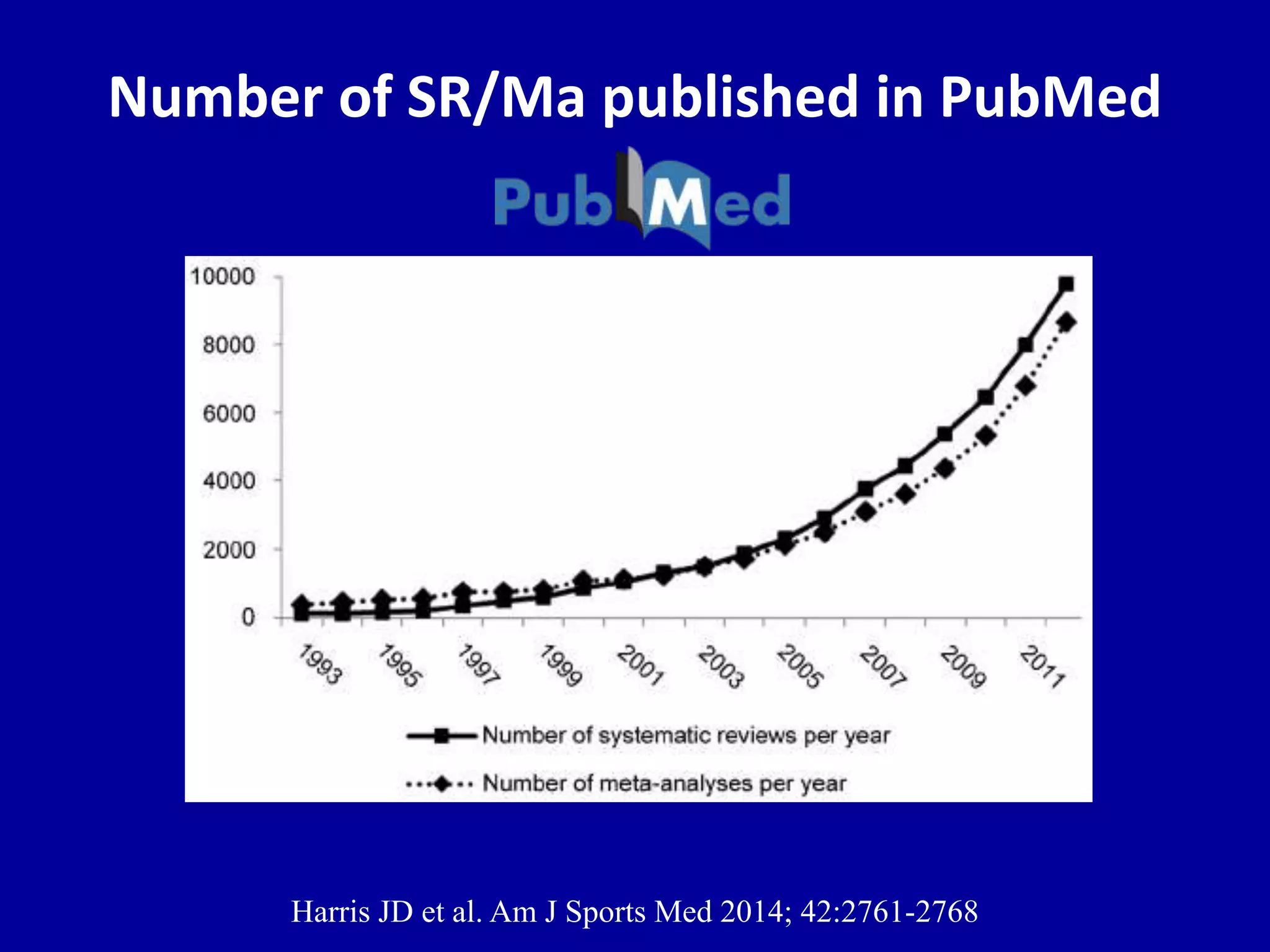
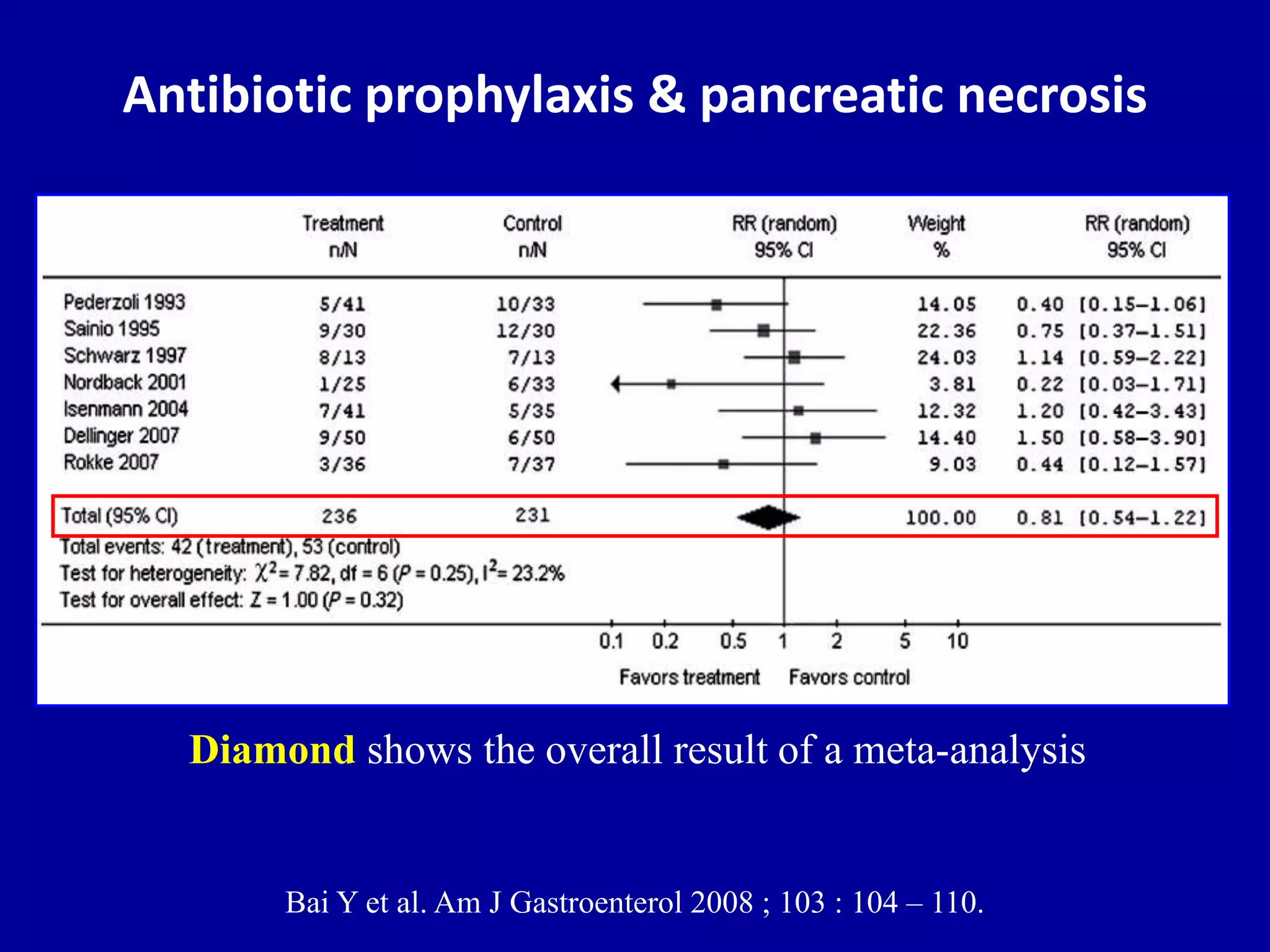
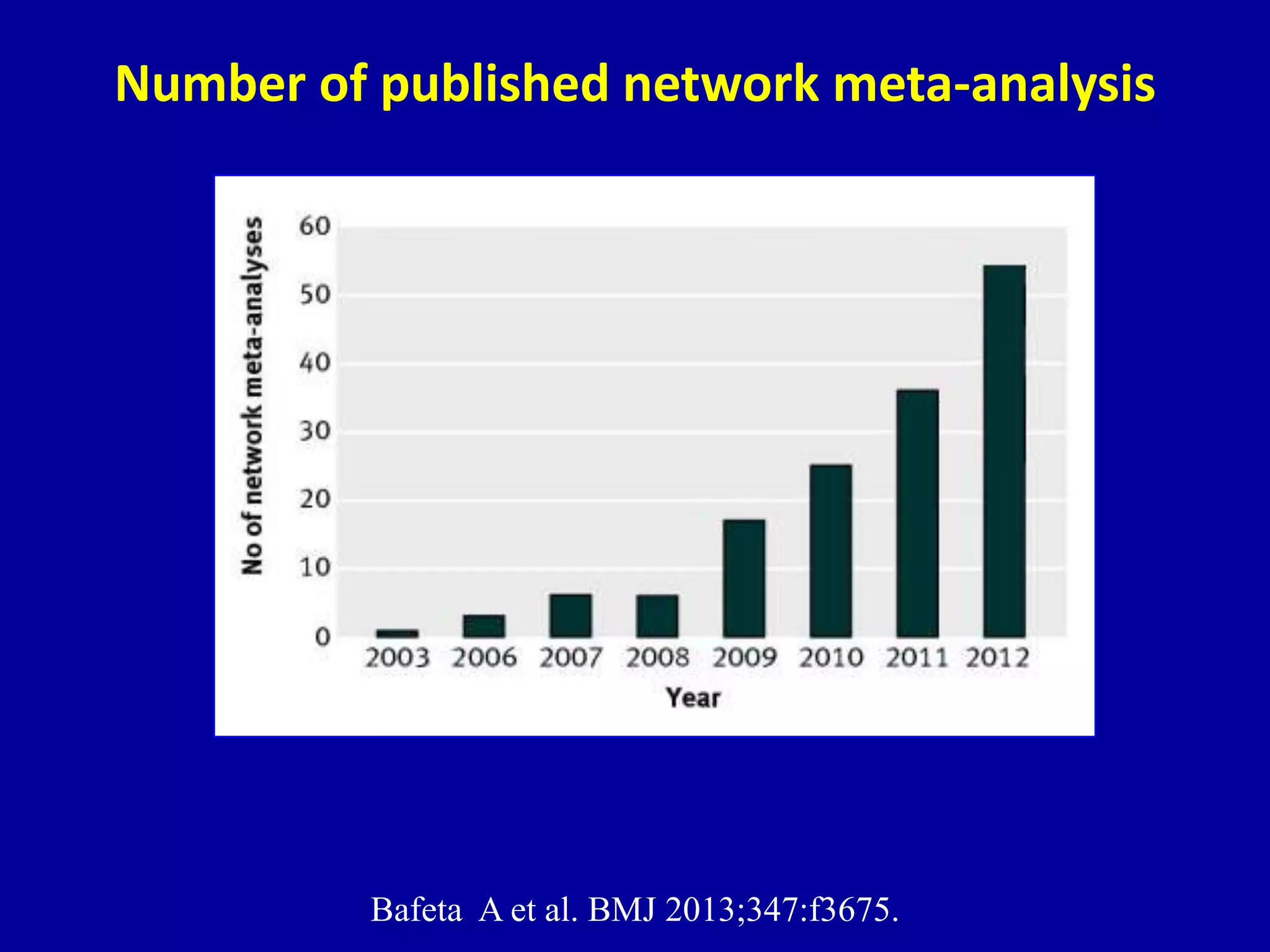
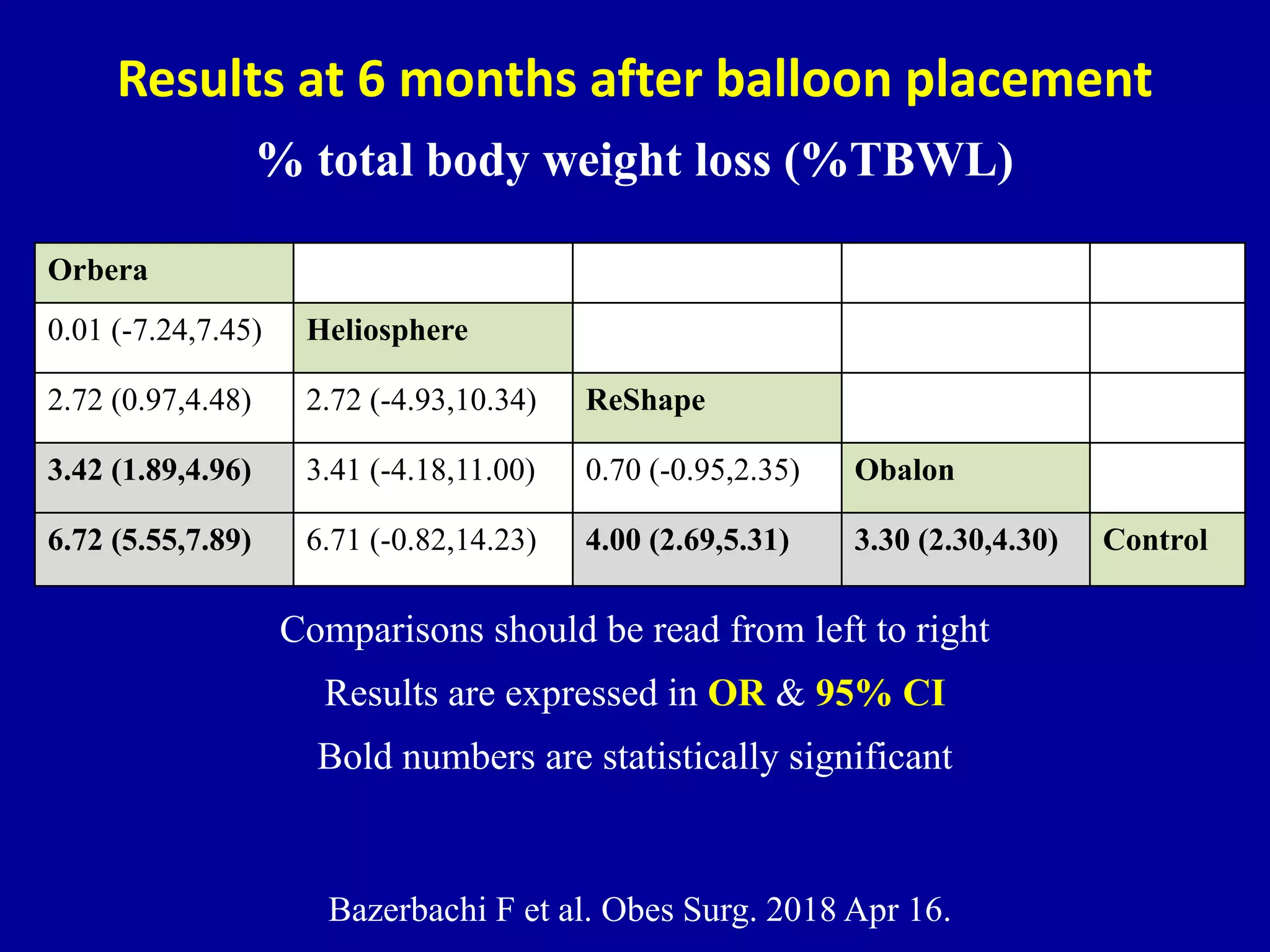
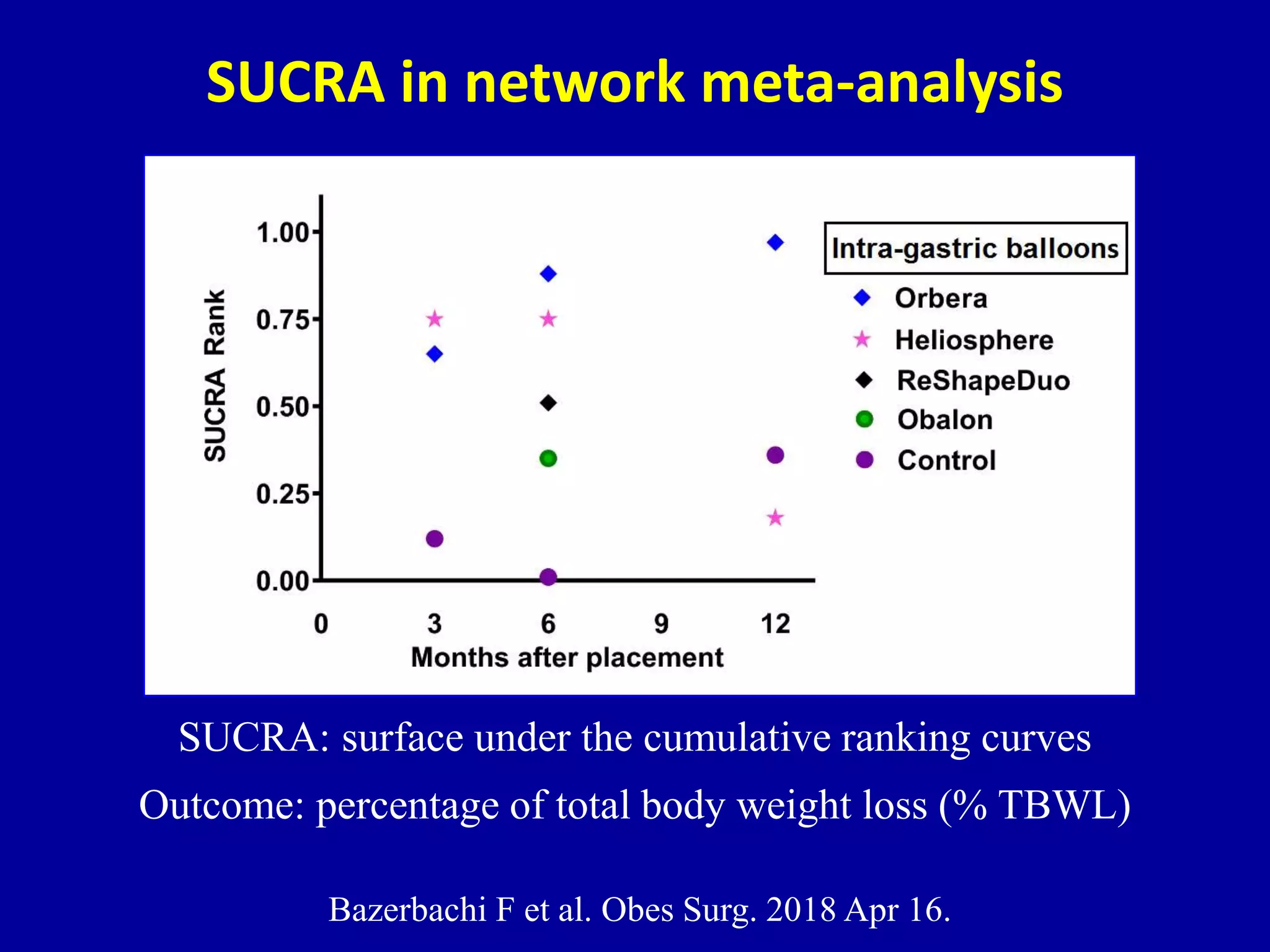
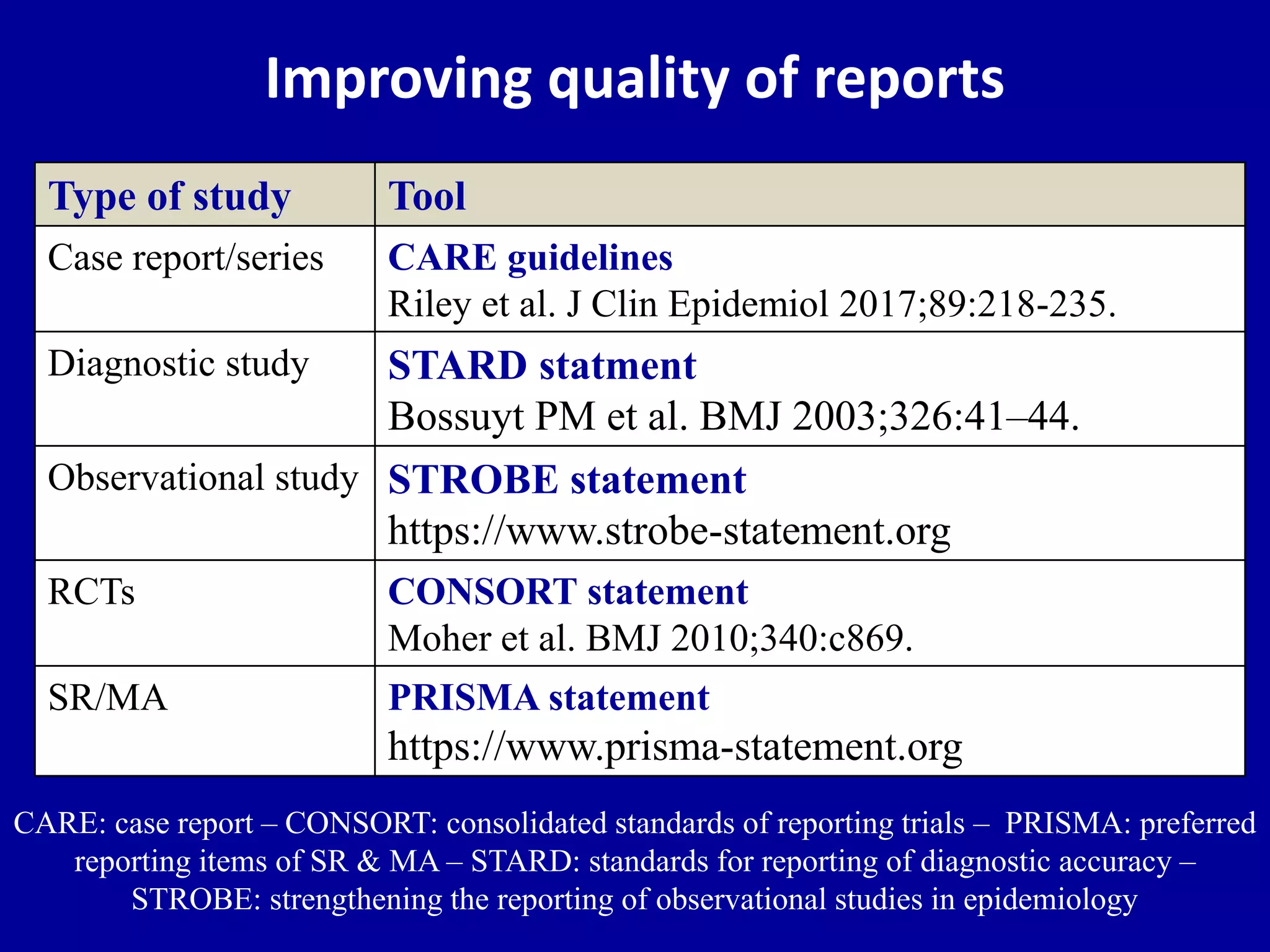
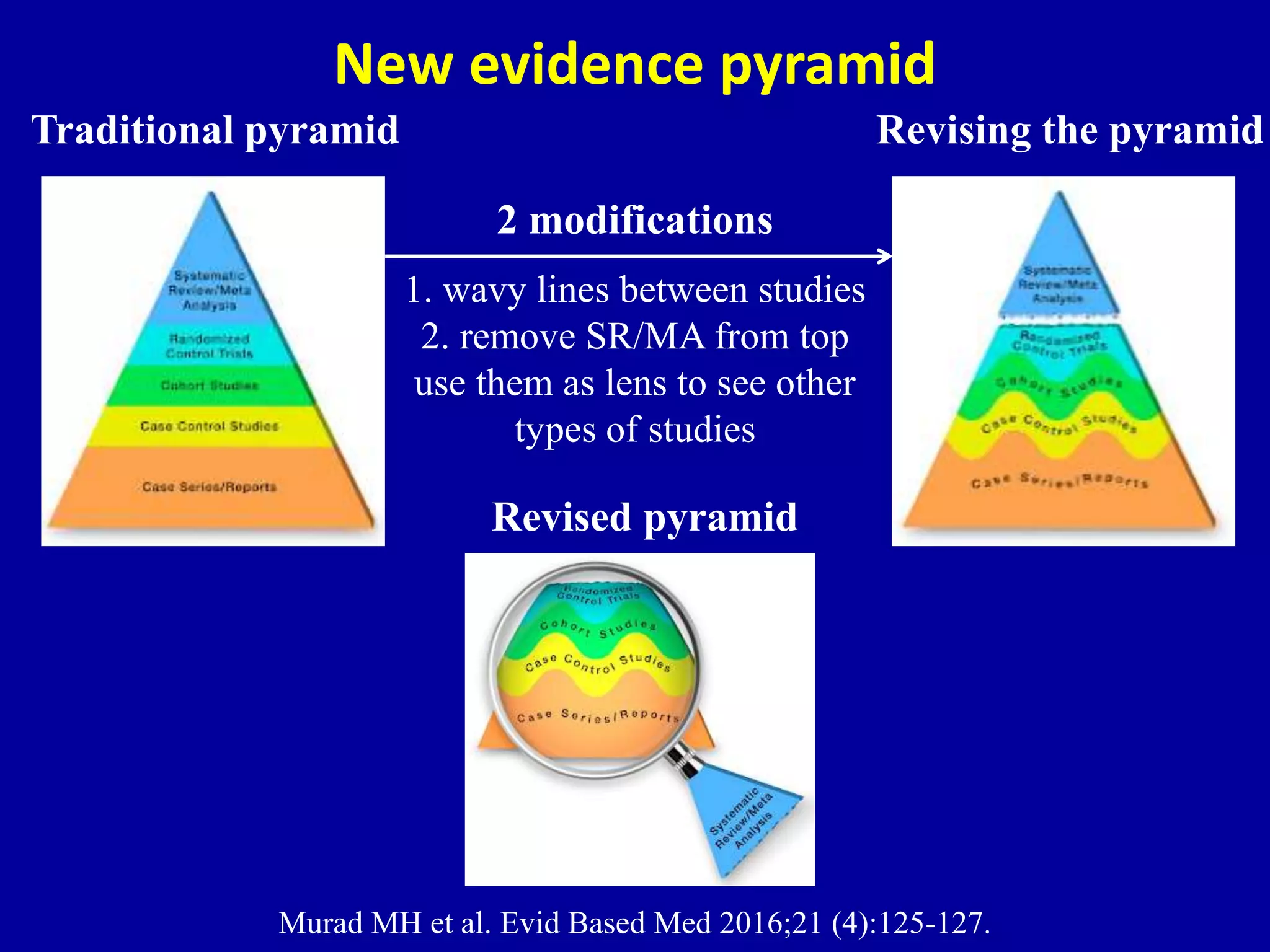
This document discusses different types of clinical studies used in evidence-based medicine, including case reports/series, ecological studies, cross-sectional studies, case-control studies, cohort studies, randomized clinical trials, systematic reviews, and meta-analyses. It provides details on study designs, strengths and limitations, and how to interpret results including risk ratios, odds ratios, confidence intervals, and p-values. Key concepts covered include biases, confounding factors, prevalence versus incidence, and how study size influences precision.