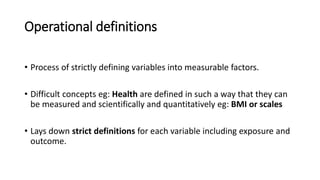
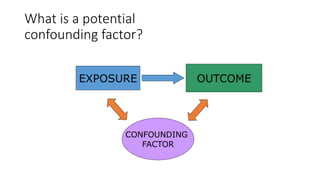
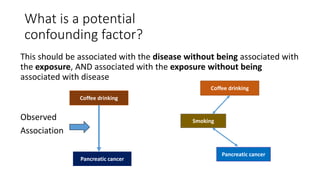
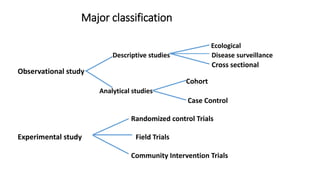
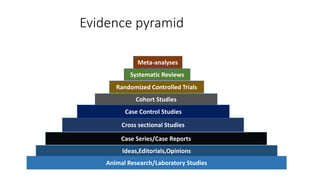
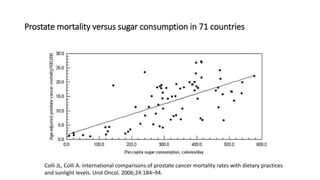
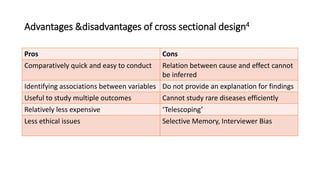
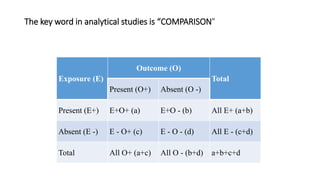
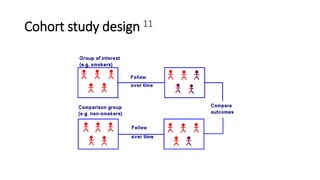
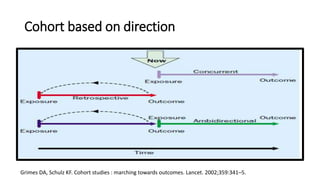
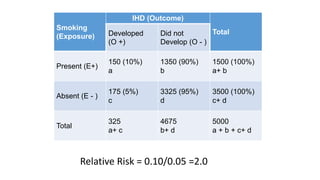
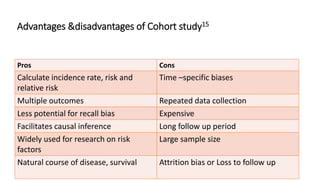
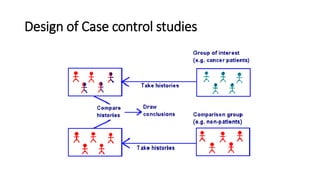
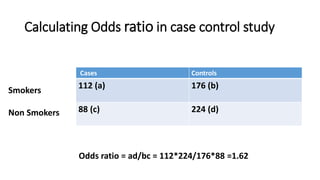
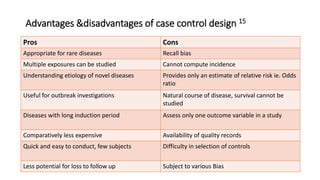
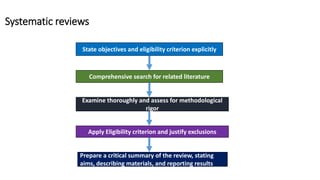
This document discusses various study designs used in epidemiology, including measures of disease occurrence such as prevalence and incidence. It defines prevalence as the total number of cases of a disease at a specified time, while incidence refers to the number of new cases that occur over a period of time. Cohort studies are described as following groups over time to compare rates of an outcome between those exposed and unexposed to a factor. Case-control studies select groups based on having or not having an outcome and look back to compare exposures. Biases such as selection, information and confounding are also outlined.