The document provides information on periodic safety update reports (PSURs), including:
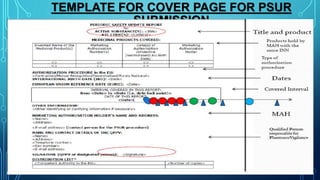
- PSURs are intended to evaluate the risk-benefit balance of a drug based on new or changing information during the post-approval phase.
- The objectives of a PSUR are to examine if new safety information aligns with previous knowledge, summarize relevant new safety data that could impact risk-benefit analysis, and provide an integrated risk-benefit evaluation.
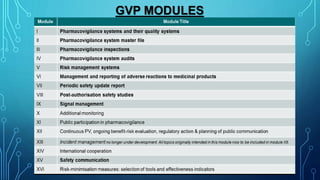
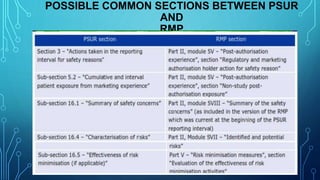
- Guidelines for PSURs are provided in the ICH E2C guideline and EU's GVP Module VII, with the format and content changing to focus more on risk-benefit analyses and summary tables rather than individual case reports.