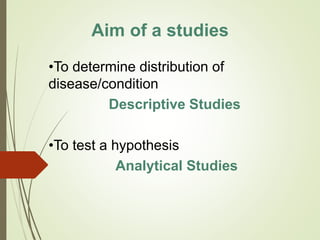
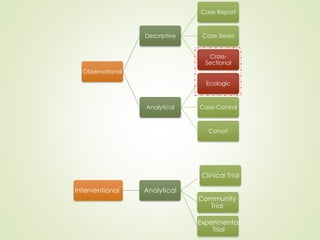
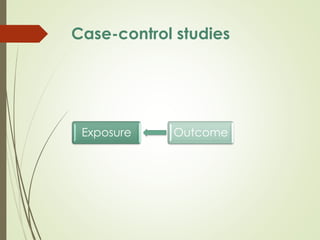
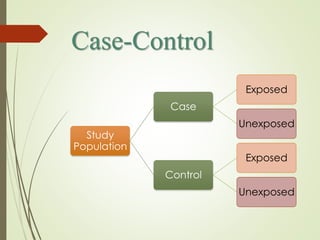
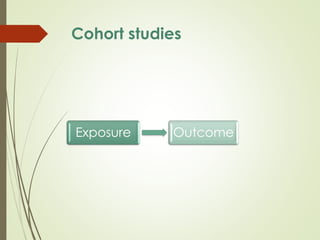
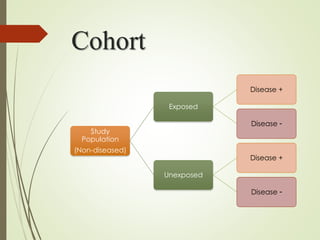
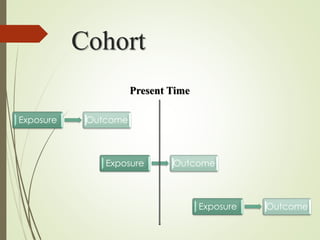
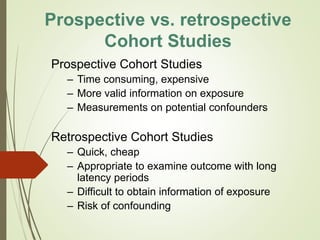
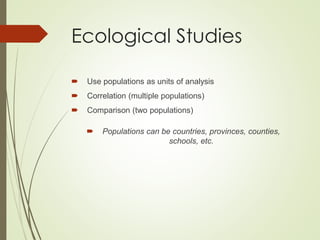
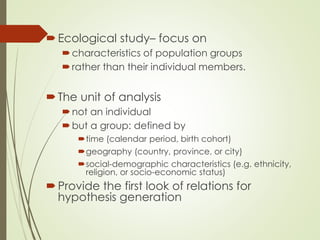
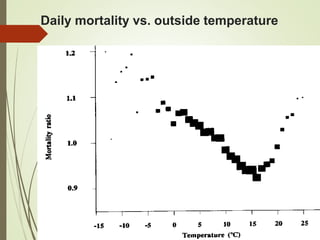
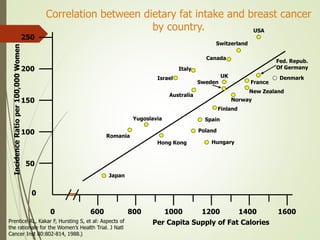
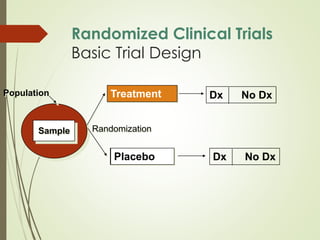
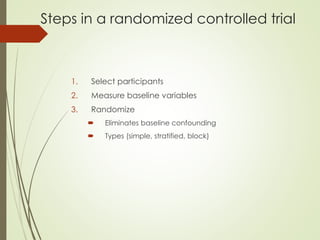
The document discusses different types of epidemiological studies, including descriptive studies like case reports and case series that focus on person, place and time to create hypotheses. Analytical studies like case-control and cohort studies are used to test hypotheses by being either observational or interventional. Randomized controlled trials are the gold standard for comparing new interventions. Observational analytical studies include cross-sectional, cohort and case-control designs, while interventional analytical studies are clinical trials. The appropriate study design depends on the research goals and objectives.