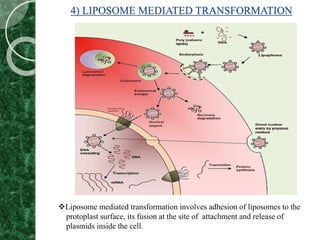
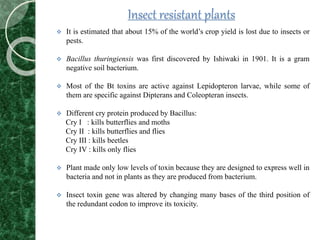
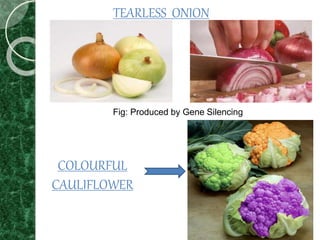
The document discusses transgenic plants, which are organisms that have undergone genetic modification by introducing foreign genes. It outlines the history and methods of transgenesis, including agrobacterium-mediated gene transfer, physical techniques like electroporation, and chemical methods such as PEG-mediated transformation. The applications of transgenic plants range from improving nutritional quality and resistance to pests and diseases to producing pharmaceuticals and enhancing crop yield.