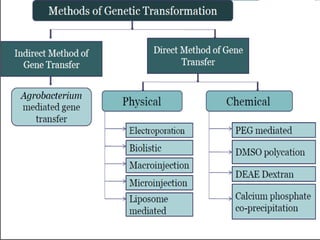
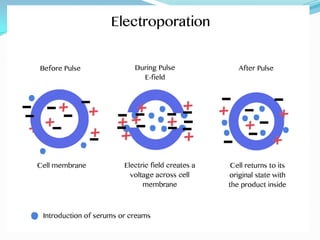
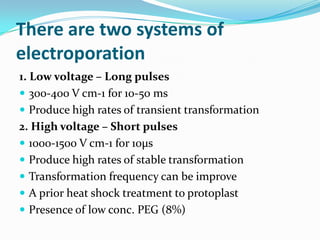
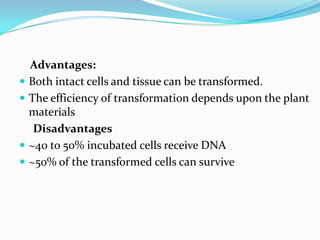
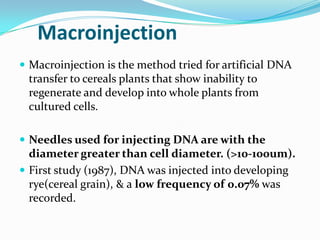
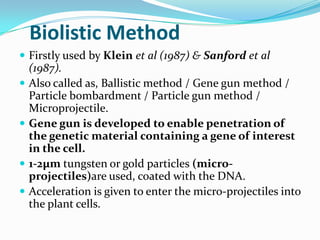
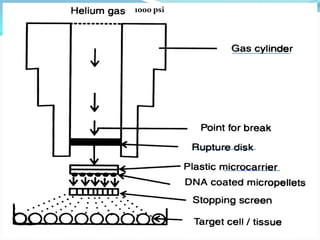
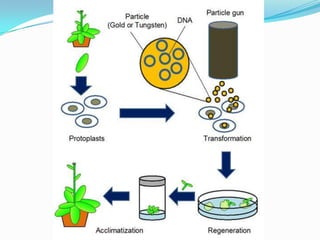
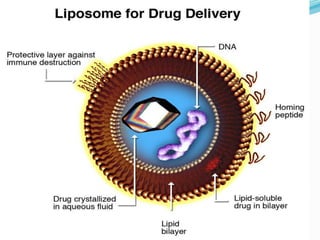
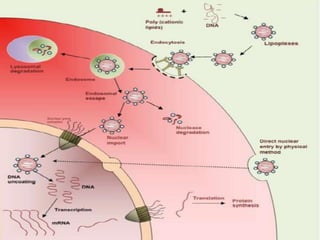
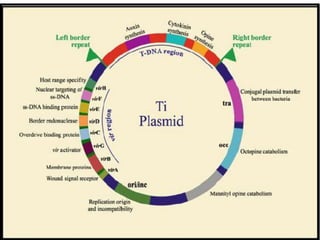
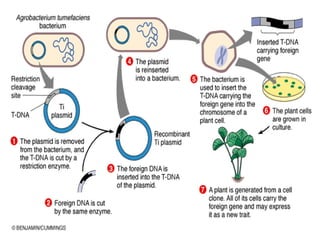
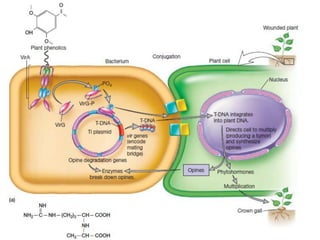
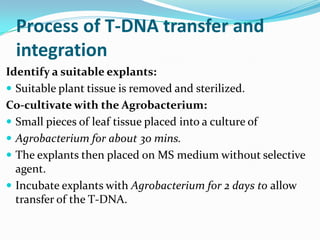
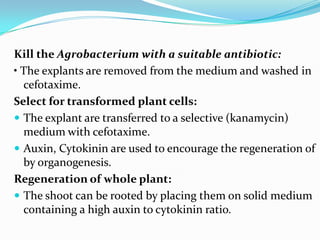
The document discusses various gene transfer methods used in plants, including Agrobacterium-mediated transfer and electroporation. It outlines the advantages and disadvantages of each technique, such as the simplicity and efficiency of methods like biolistics and liposome-mediated transfer, alongside their limitations. Overall, the detailed examination provides insights into the processes of DNA integration and the challenges associated with plant transformation.