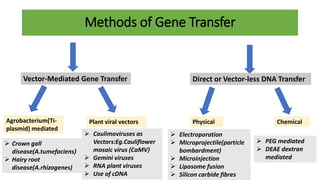
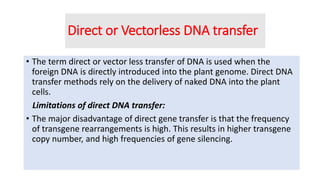
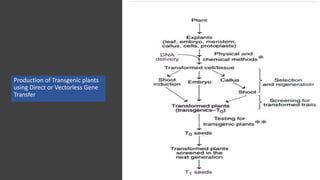
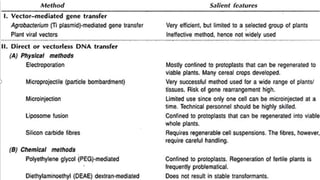
The document discusses gene transfer methods in plants, highlighting the importance of inserting foreign genes to confer desired traits. It includes methods such as agrobacterium-mediated transfer, virus-mediated transfer, and direct DNA transfer, along with their applications like producing transgenic plants and secondary metabolites. Additionally, it emphasizes the roles of secondary metabolites in plant interactions with their environment and outlines applications of plant tissue culture for producing various compounds.