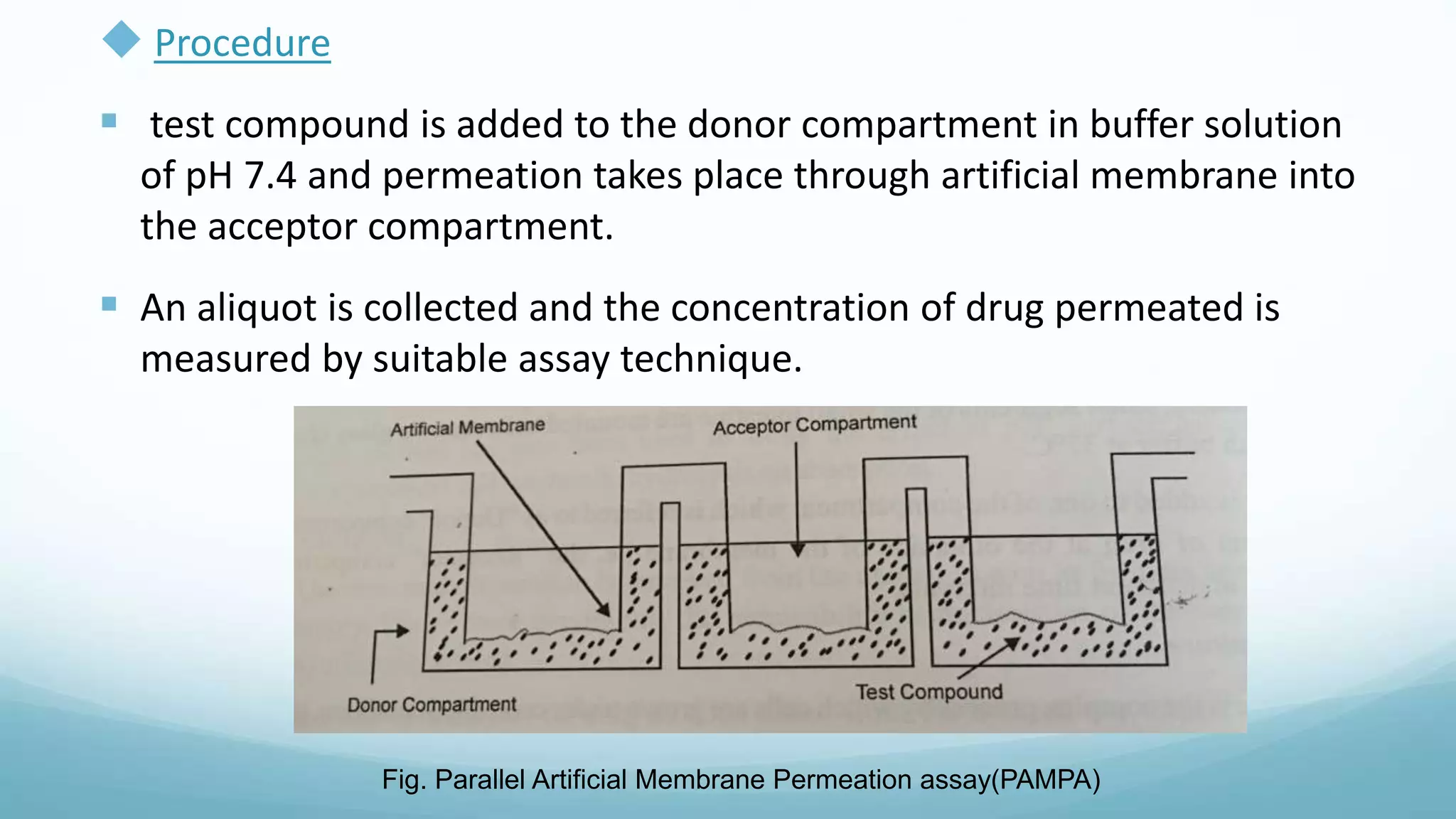
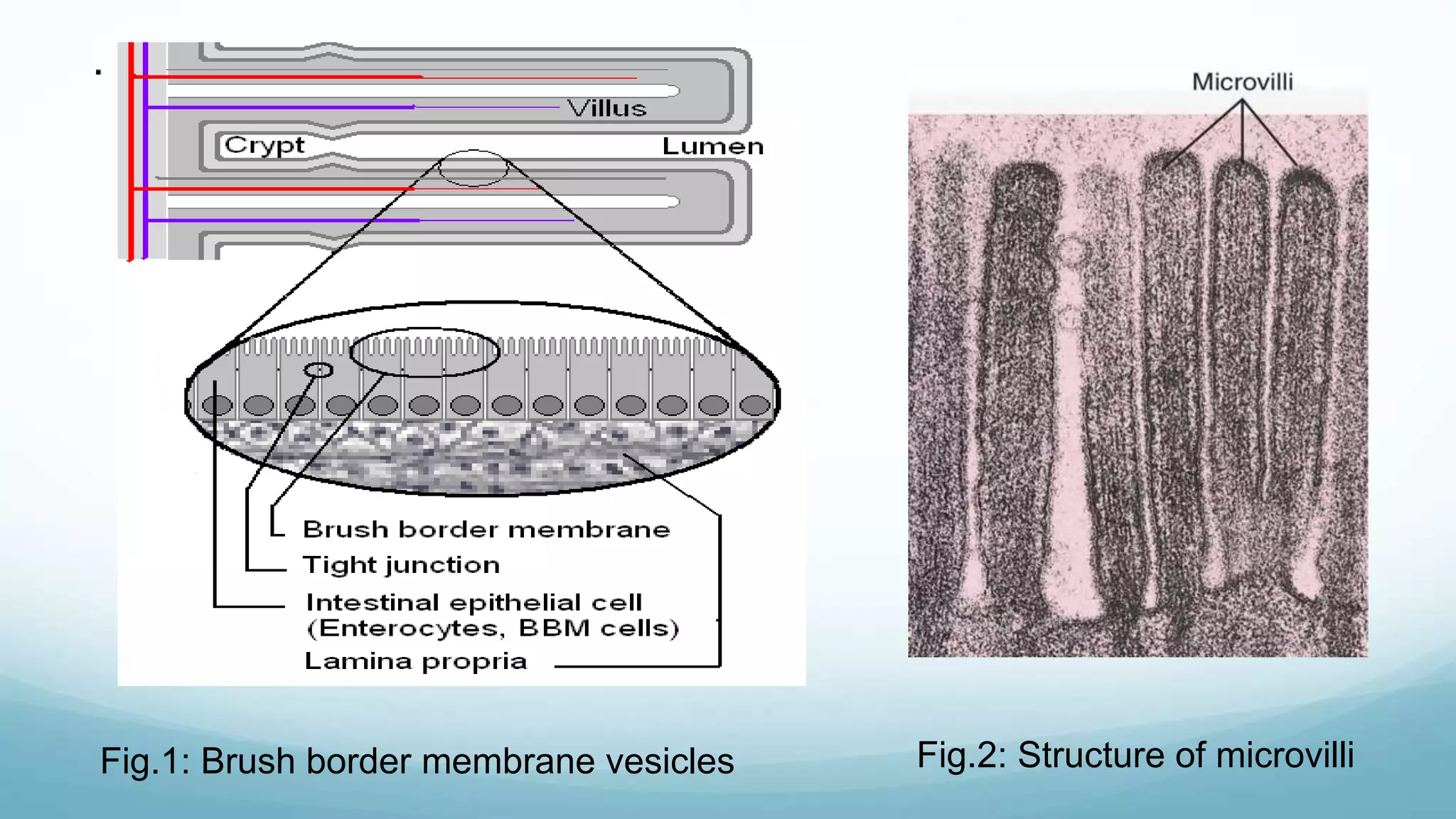
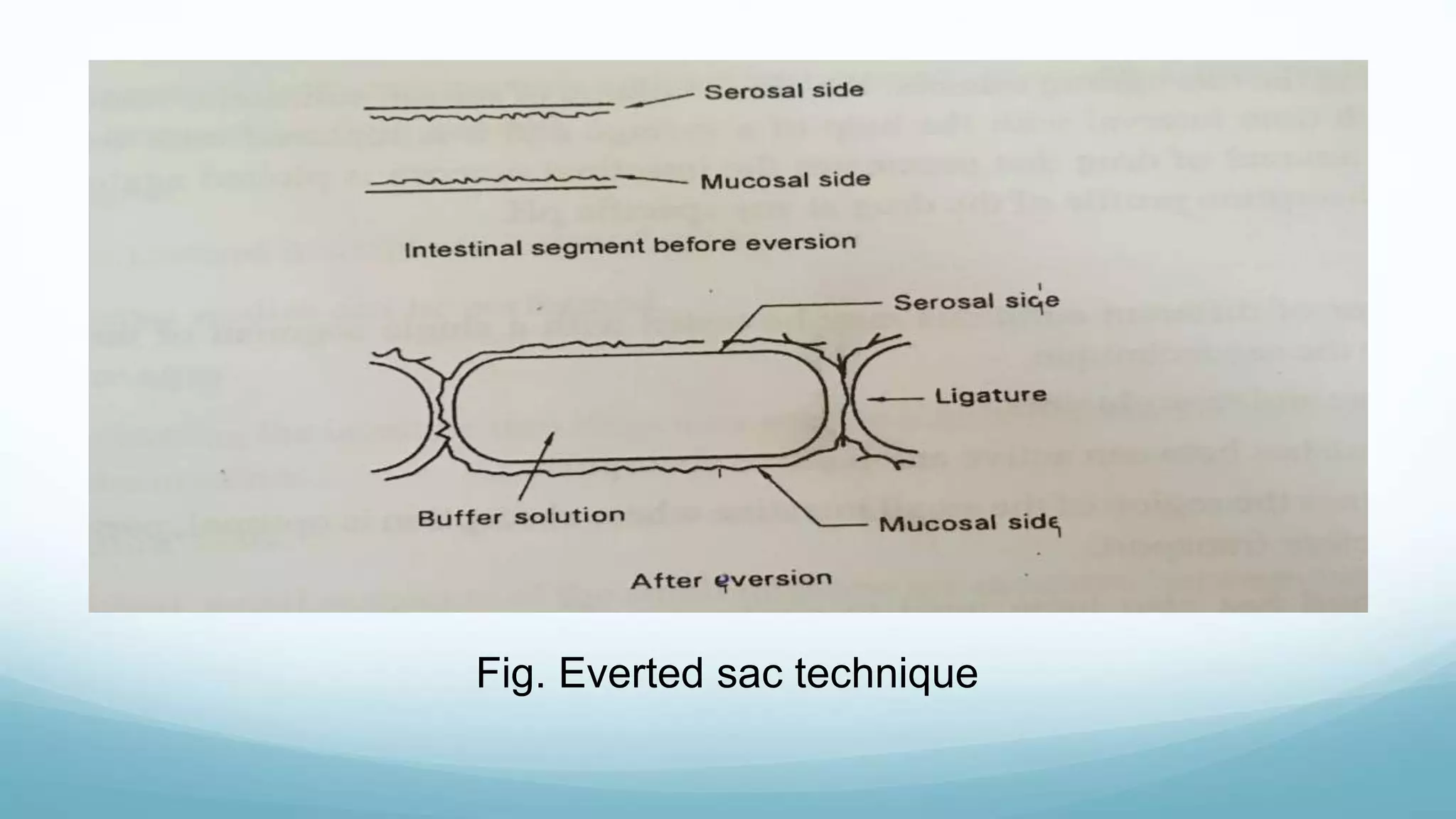
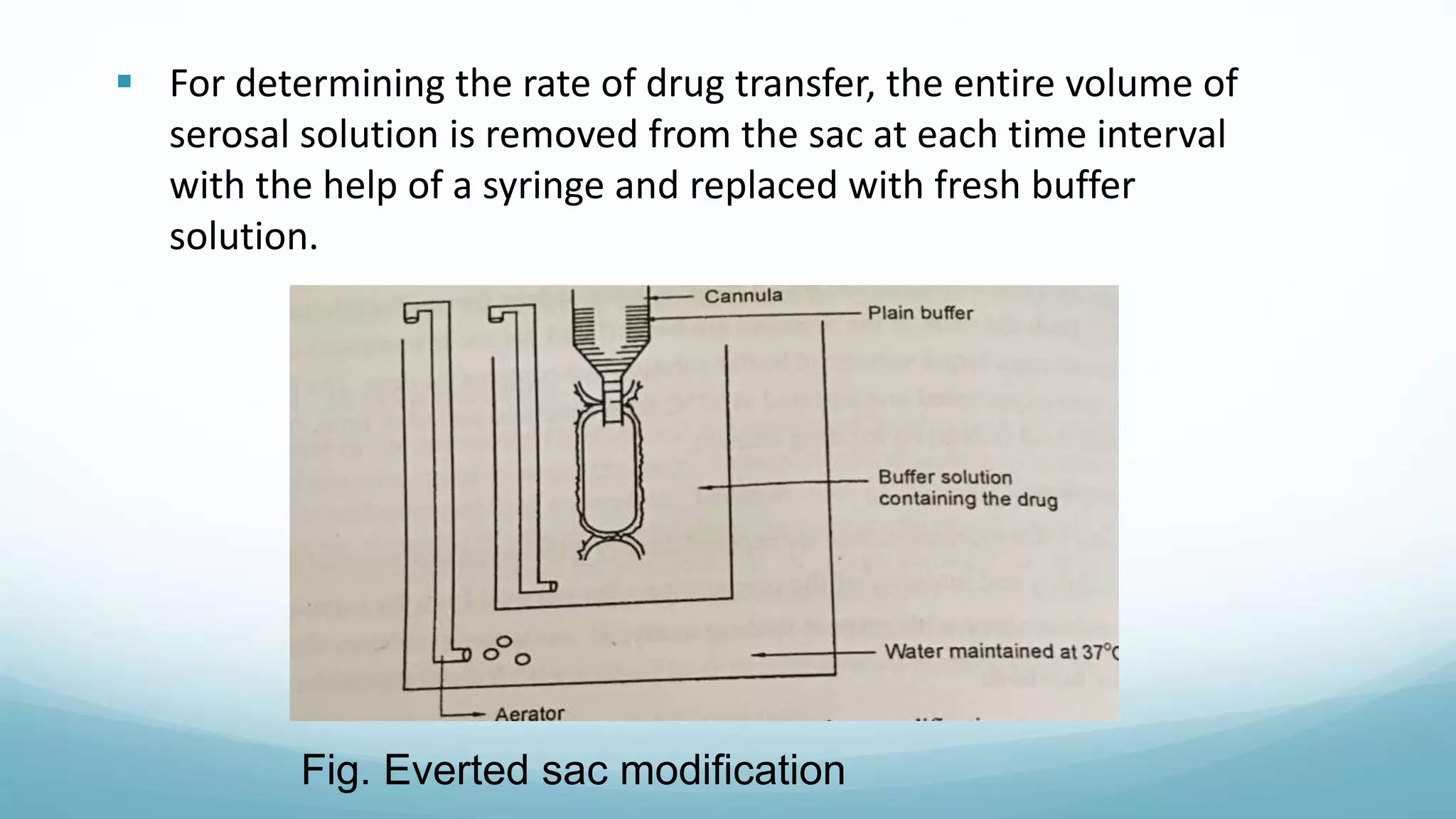
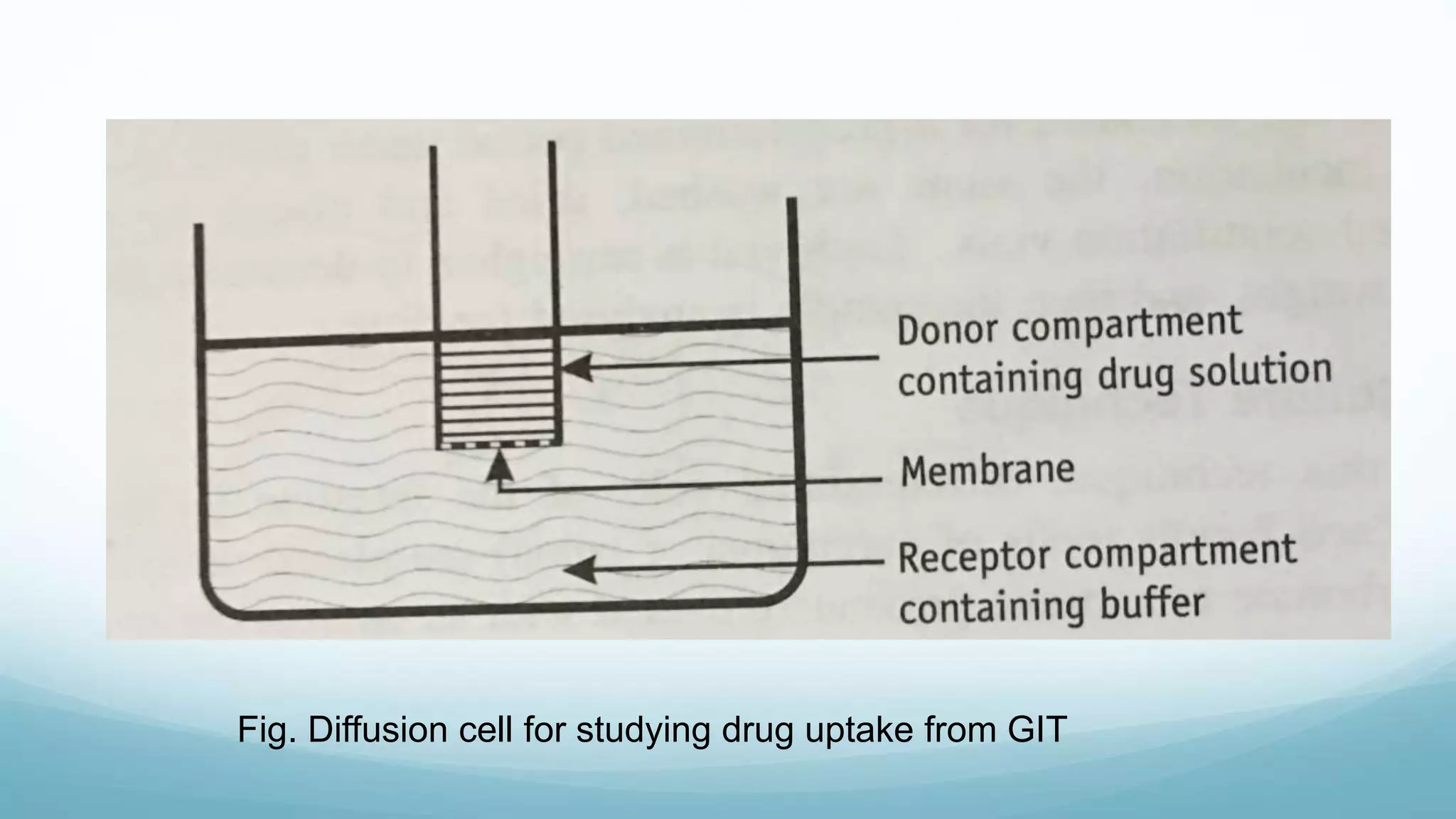
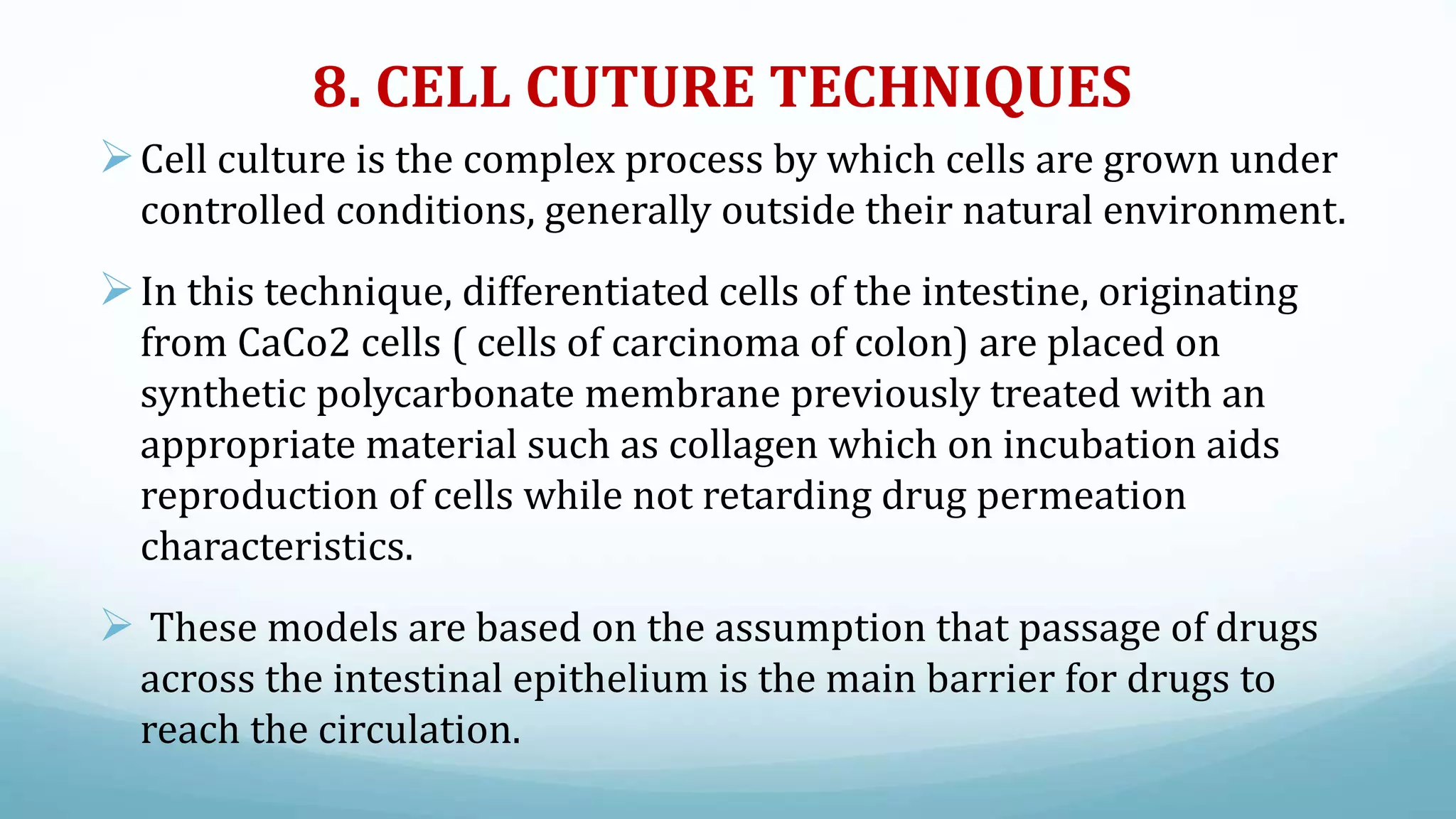
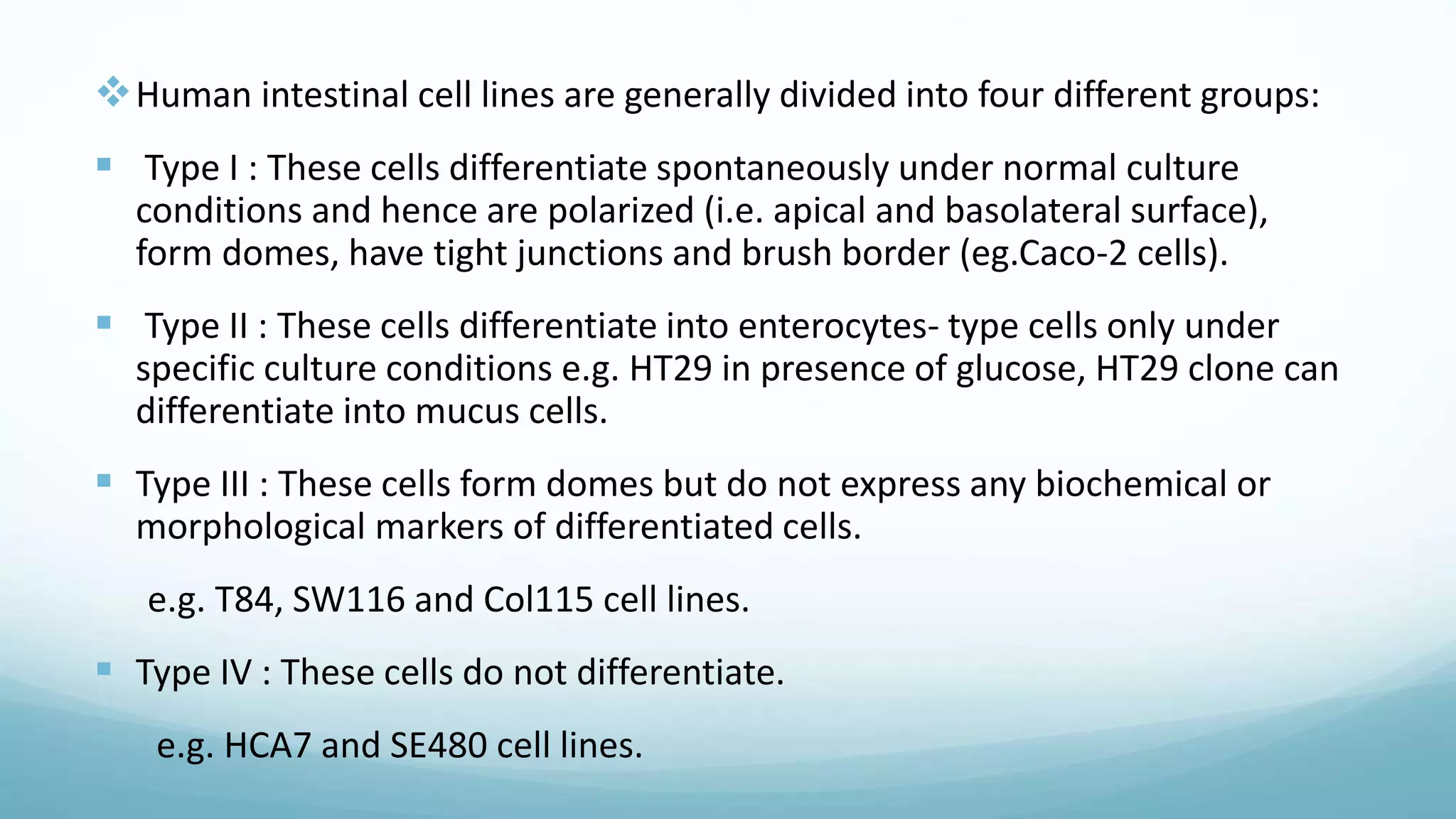
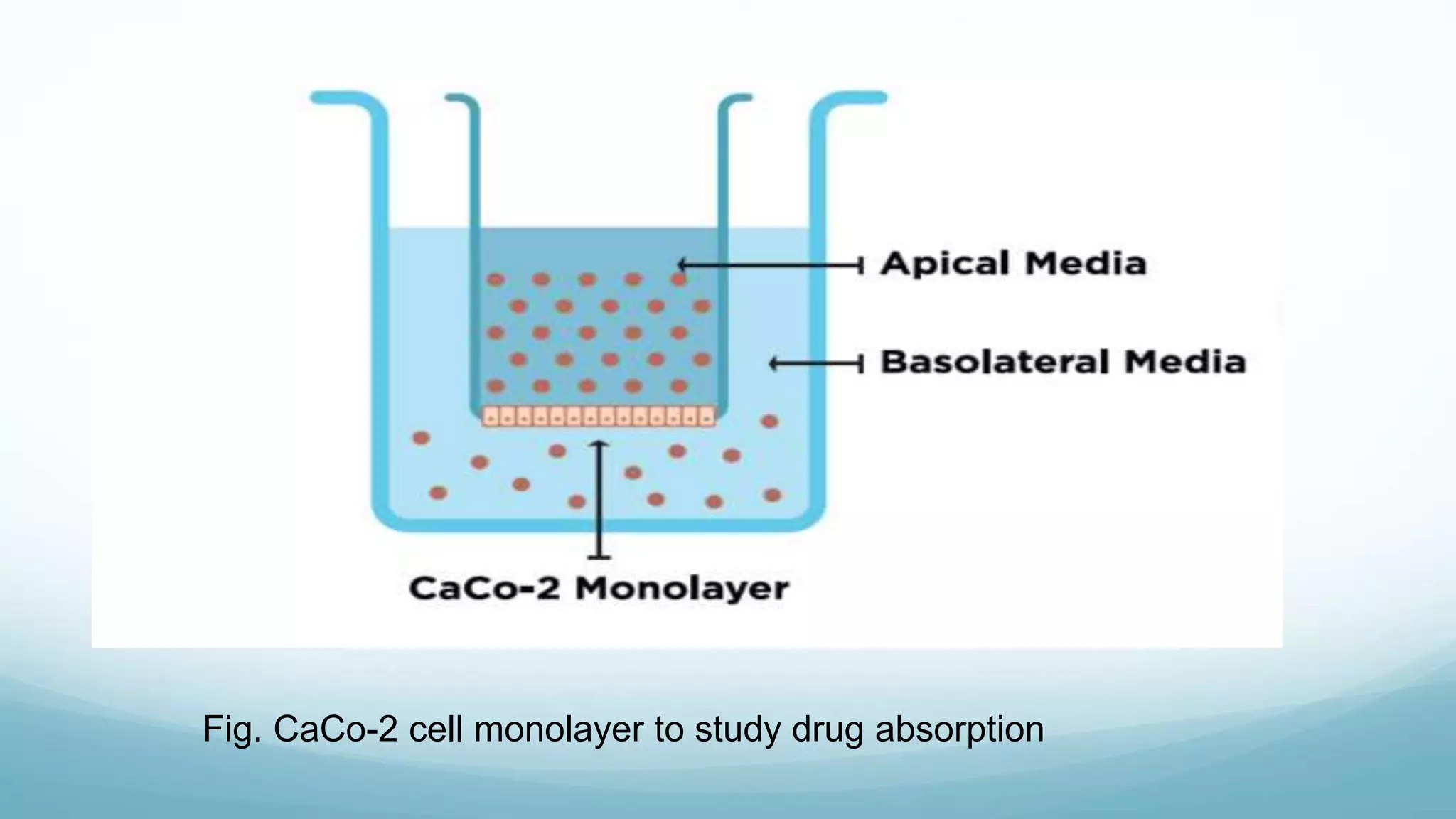
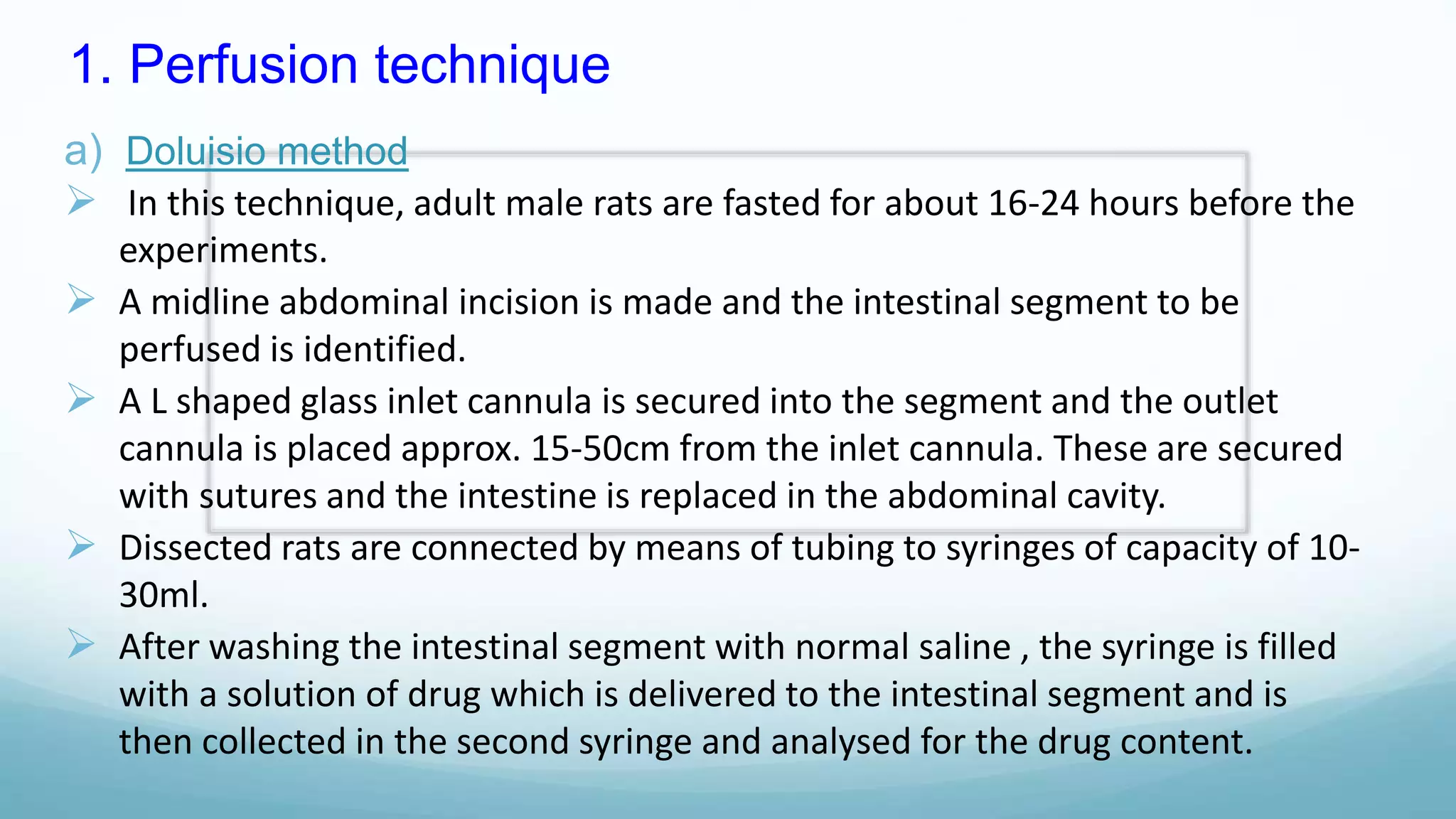
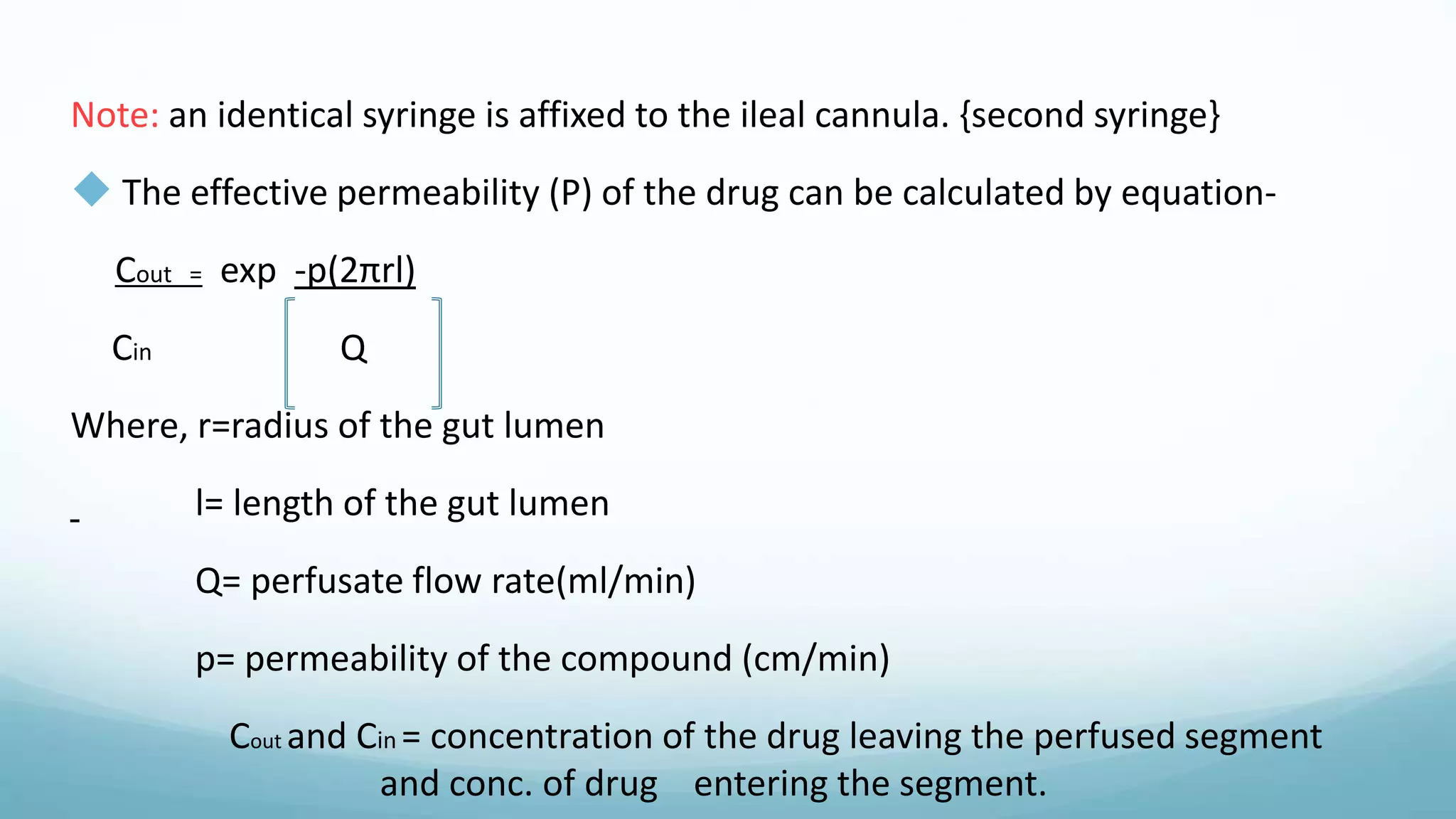
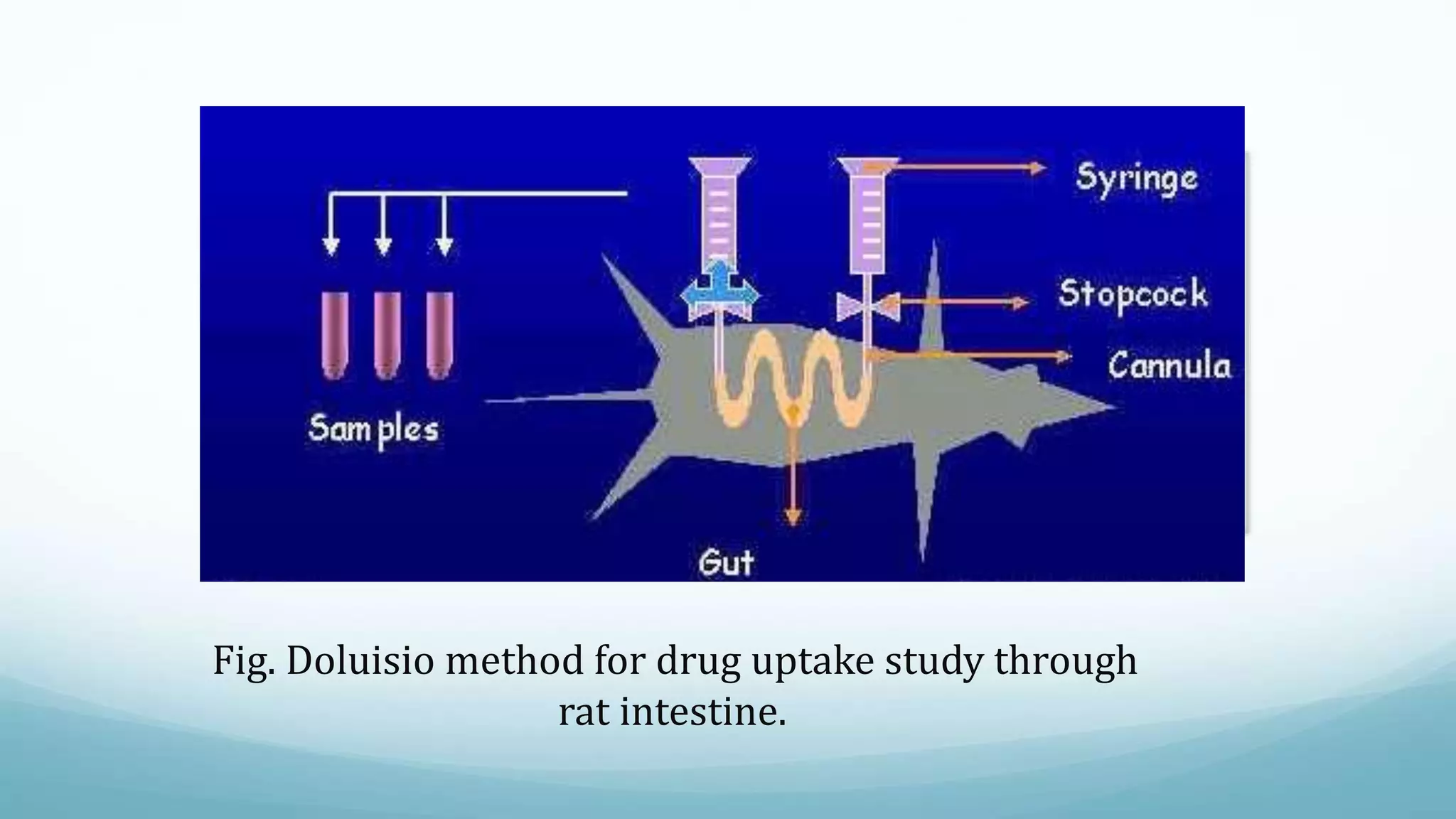
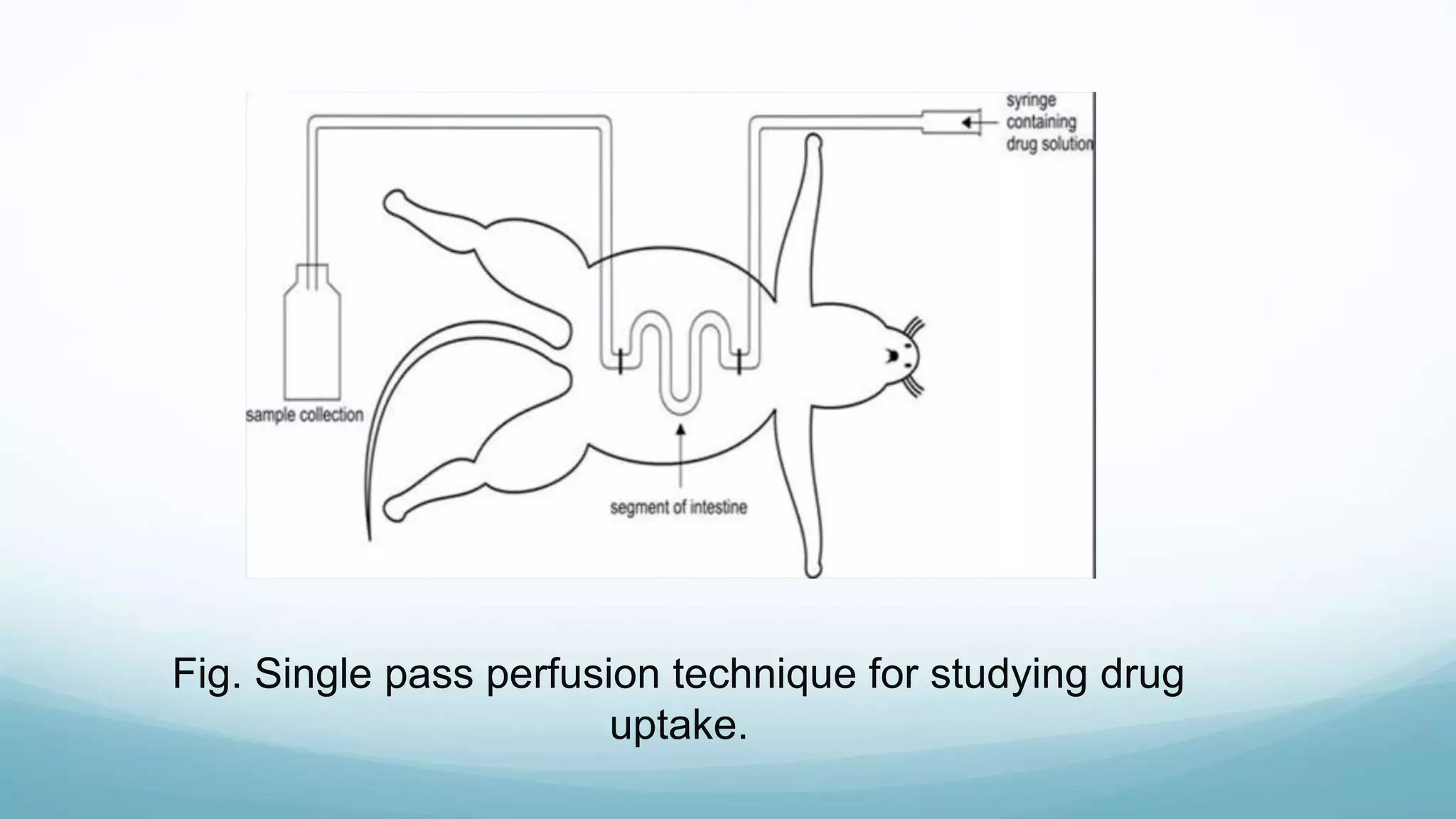
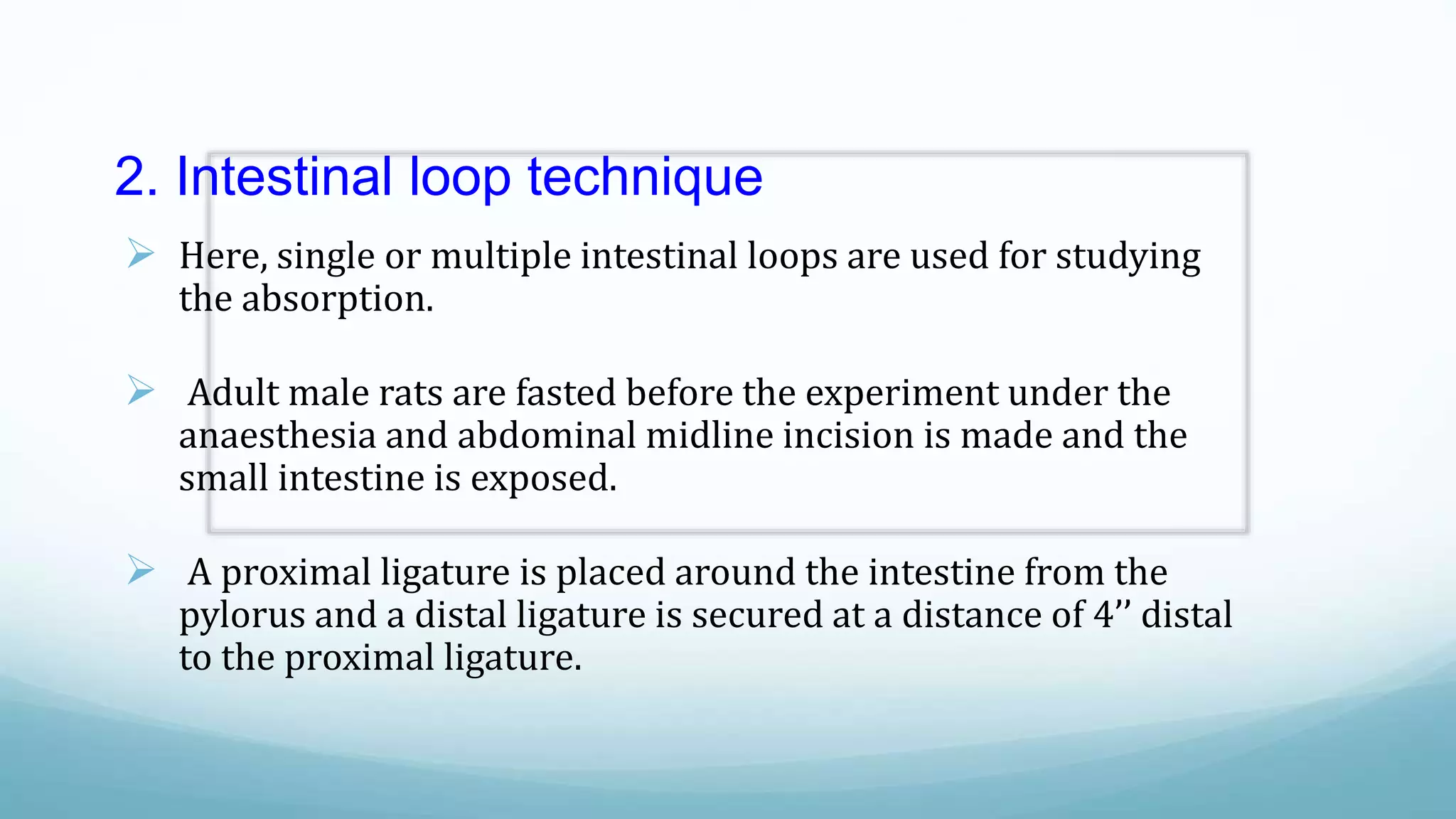
The document discusses various in vitro, in vivo, and in situ methods for determining drug absorption. In vitro methods include physicochemical techniques like measuring partition coefficients as well as more complex methods like using everted intestinal sacs or cell culture models. In vivo methods directly measure drug levels in blood/urine over time or indirectly via pharmacological response. In situ techniques simulate in vivo conditions by perfusing drug solutions through an intestinal segment while keeping the blood supply intact. The document provides detailed descriptions of techniques like the everted sac method, Caco-2 cell cultures, and Doluisio intestinal perfusion method.