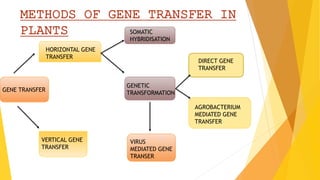
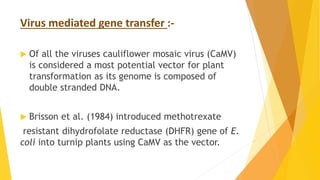
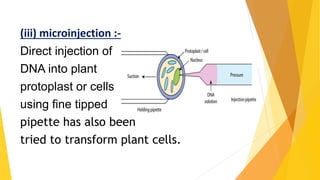
This document discusses different methods of genetic transformation in plants, including Agrobacterium-mediated gene transfer, virus-mediated gene transfer, and direct gene transfer methods like microinjection and particle bombardment. It provides details on the mechanisms of Agrobacterium-mediated transformation using Ti and Ri plasmids and describes applications of transgenic plants for herbicide resistance and insect resistance by introducing genes for Bt toxins or trypsin inhibitors.