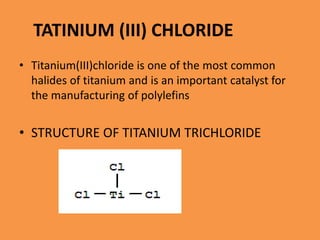
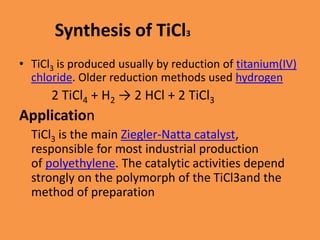
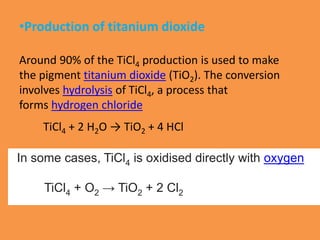
Titanium chloride encompasses various inorganic compounds including titanium dichloride, trichloride, and tetrachloride, with uses in catalysis and production of titanium metal and titanium dioxide. Titanium trichloride serves as a key Ziegler-Natta catalyst for polyethylene production and is useful in organic synthesis, while titanium tetrachloride serves as an intermediate in titanium metal production. Both compounds have distinct properties and synthesis methods, with specific applications in industrial processes.








![TITANIUM CHLORIDE [PHARMACEUTICAL REAGENT]](https://image.slidesharecdn.com/66-191220064235/85/TITANIUM-CHLORIDE-PHARMACEUTICAL-REAGENT-12-320.jpg)