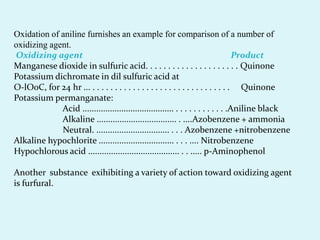
The document discusses the oxidation of aniline and compares various oxidizing agents such as manganese dioxide, potassium dichromate, and hydrogen peroxide, highlighting their stability and uses. It notes the applications of sodium hypochlorite as a bleach and disinfectant and emphasizes the commercial use of ozone for the oxidation of oleic acid. Additionally, it outlines various chemical transformations involving other organic substances and their industrial applications.