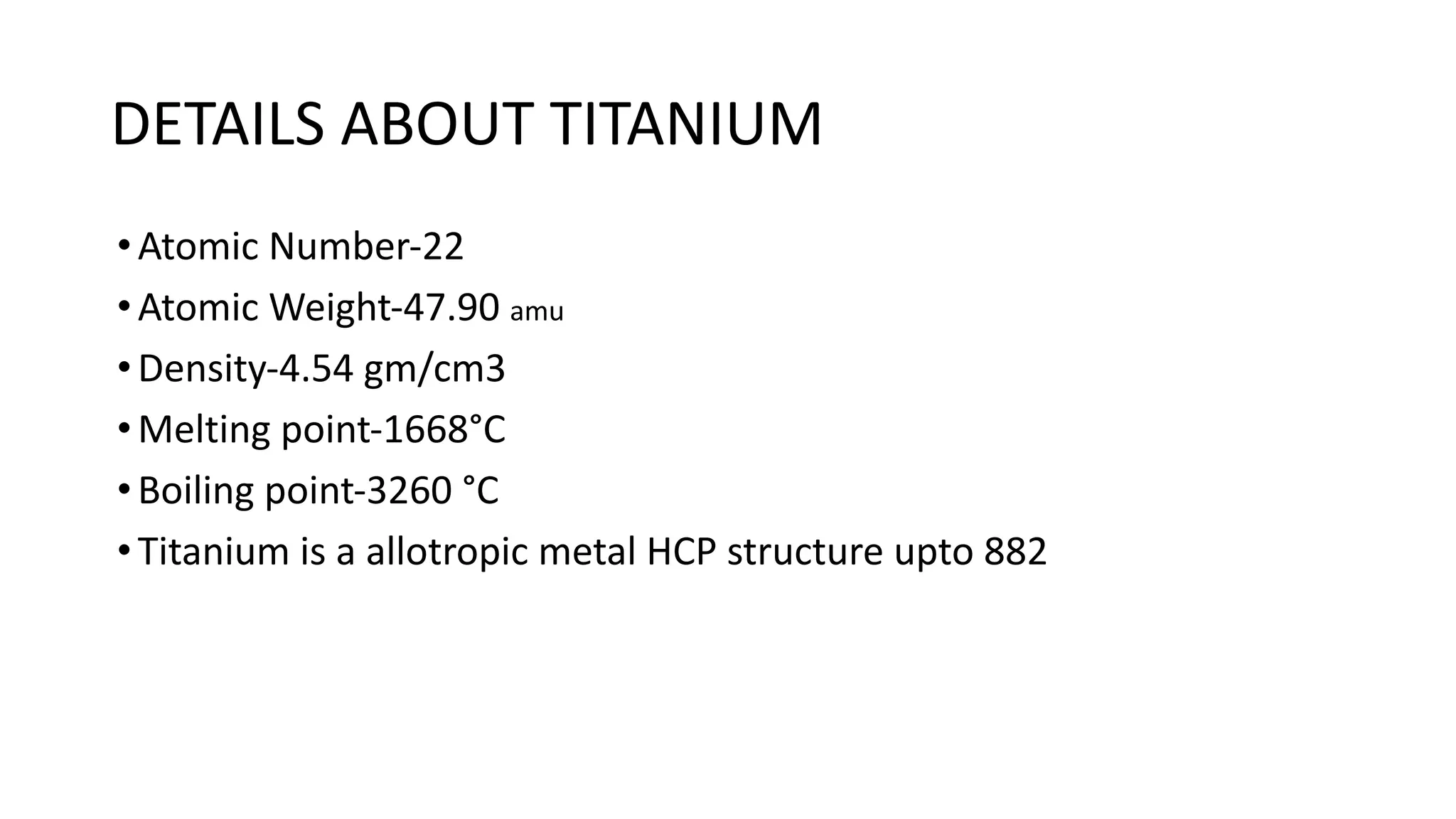
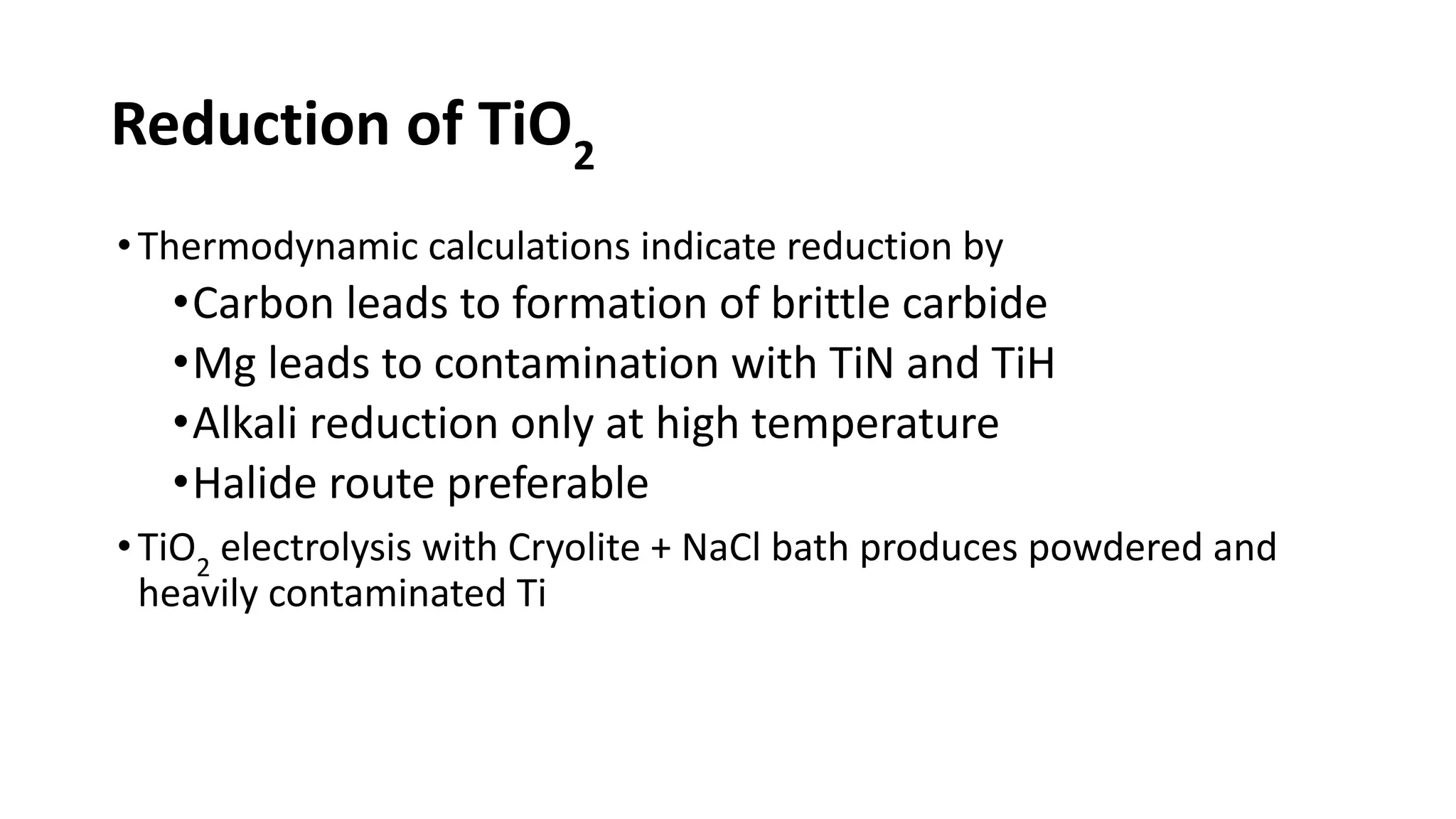
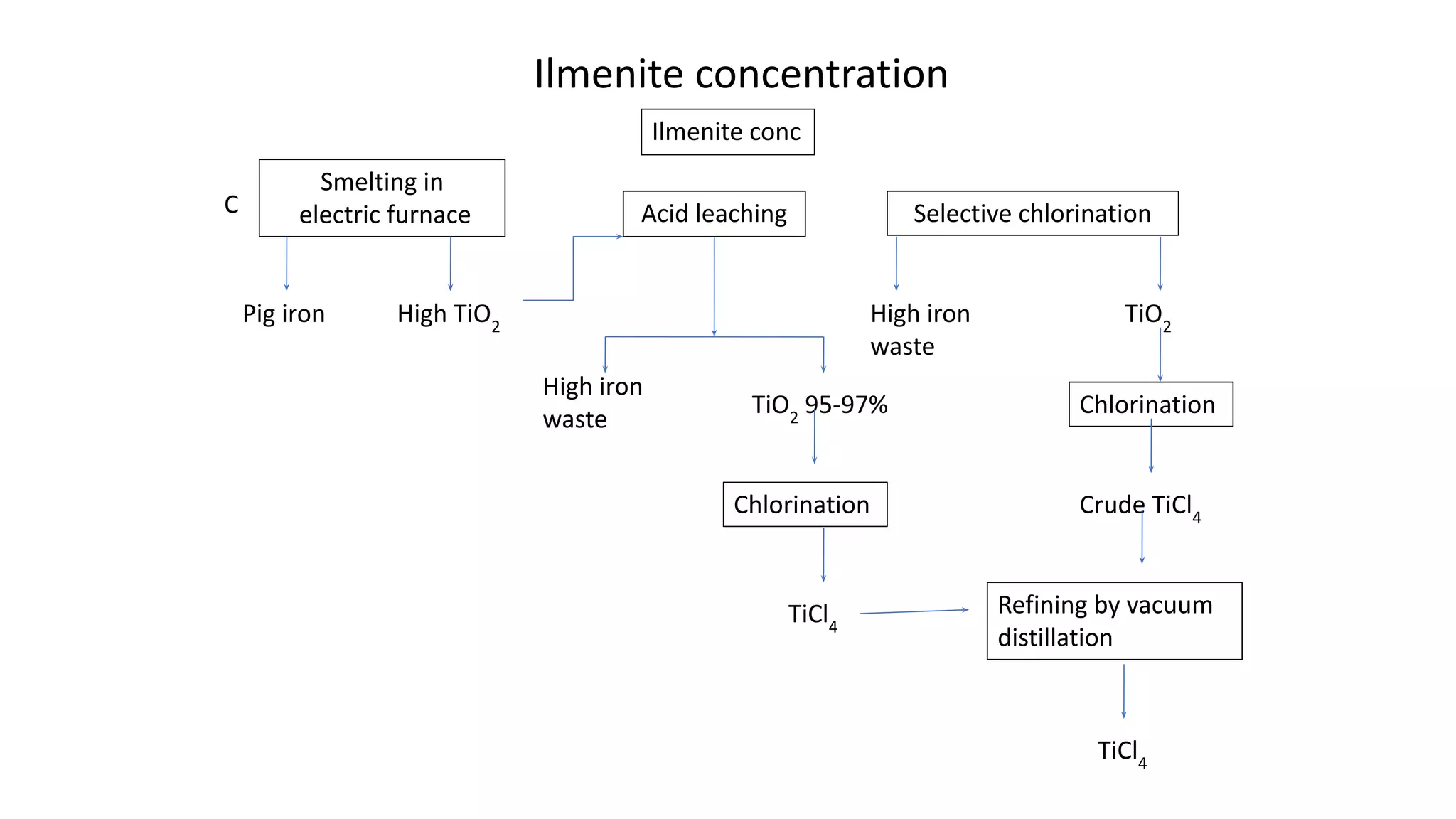
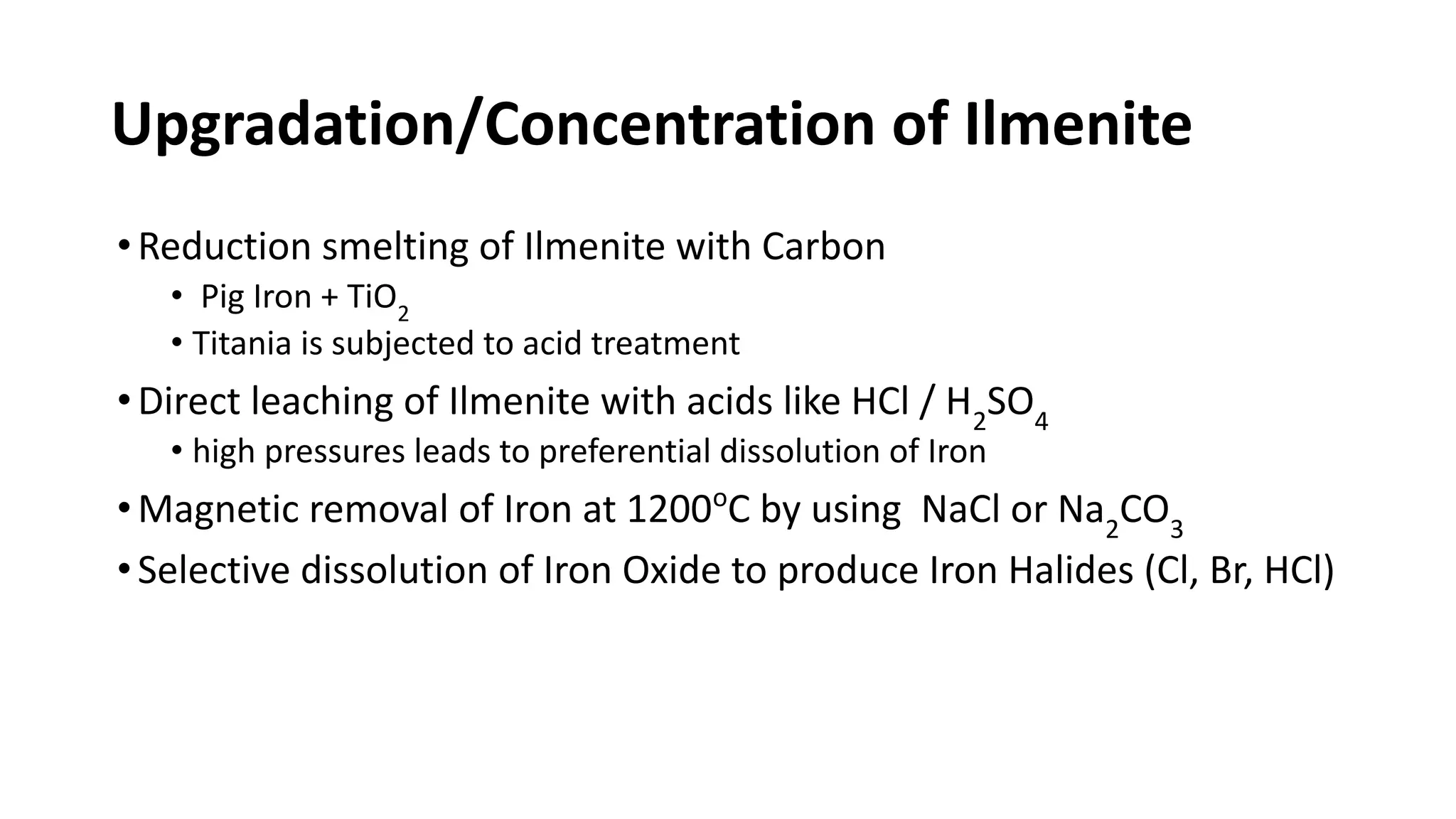
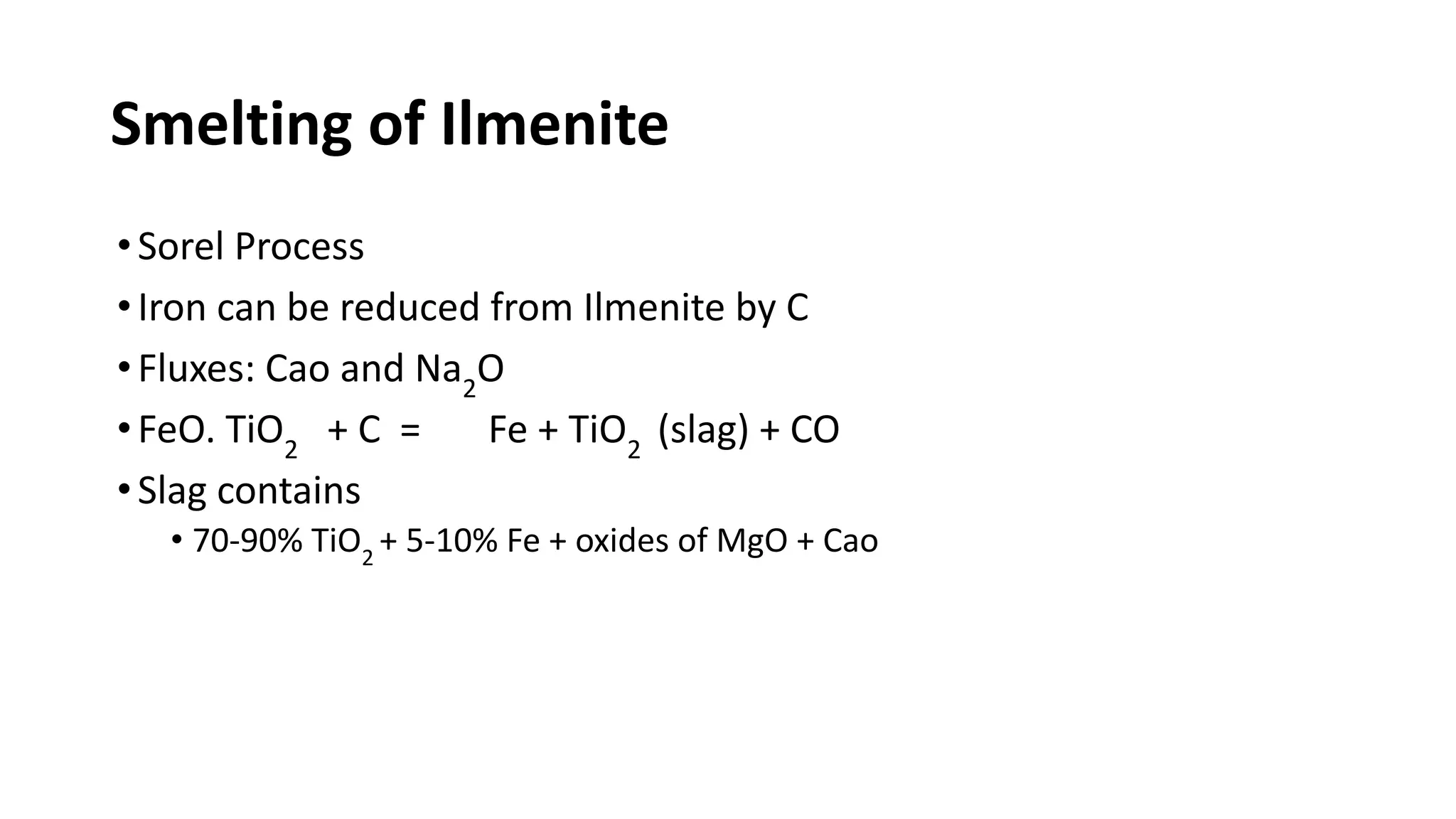
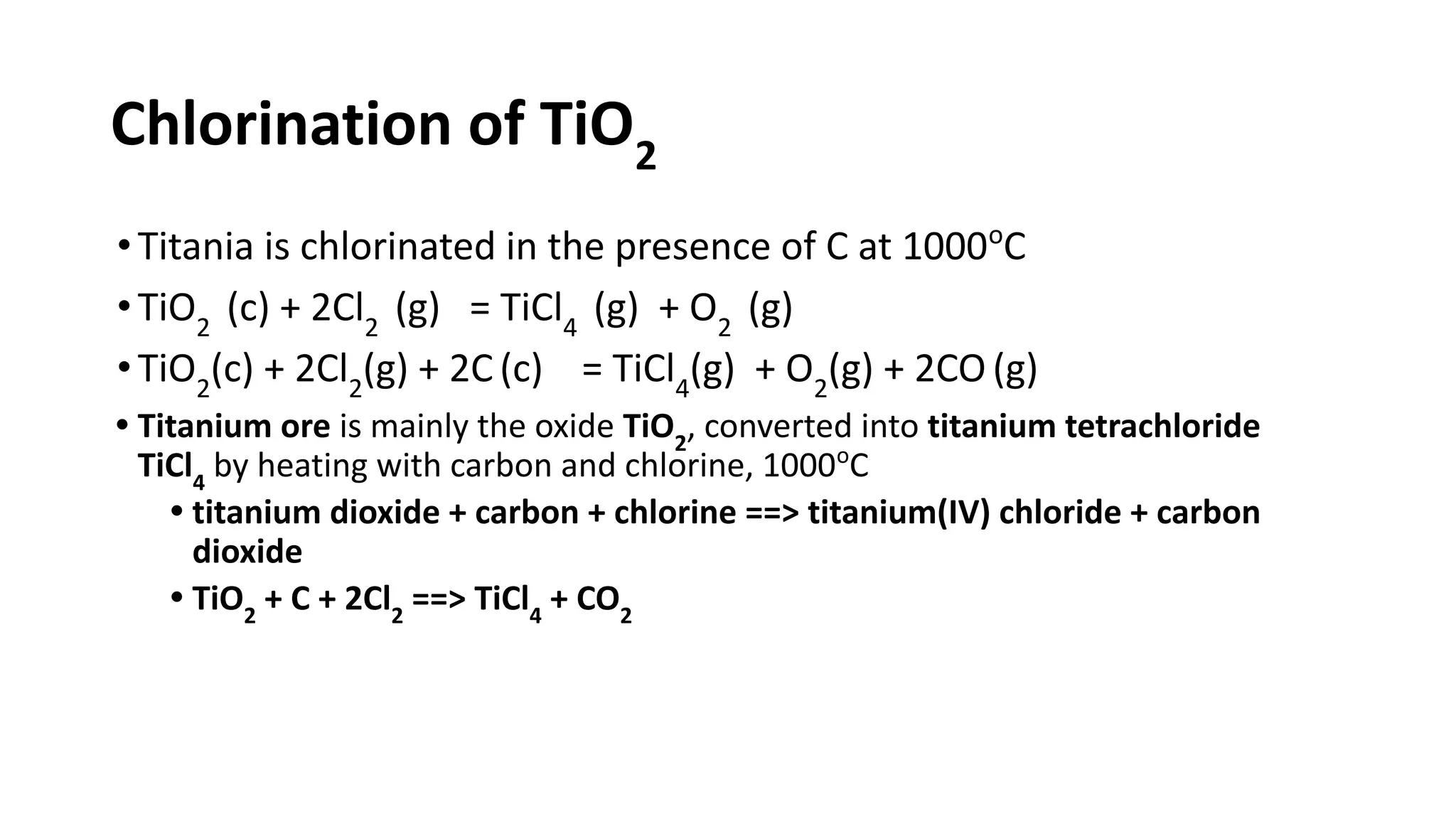
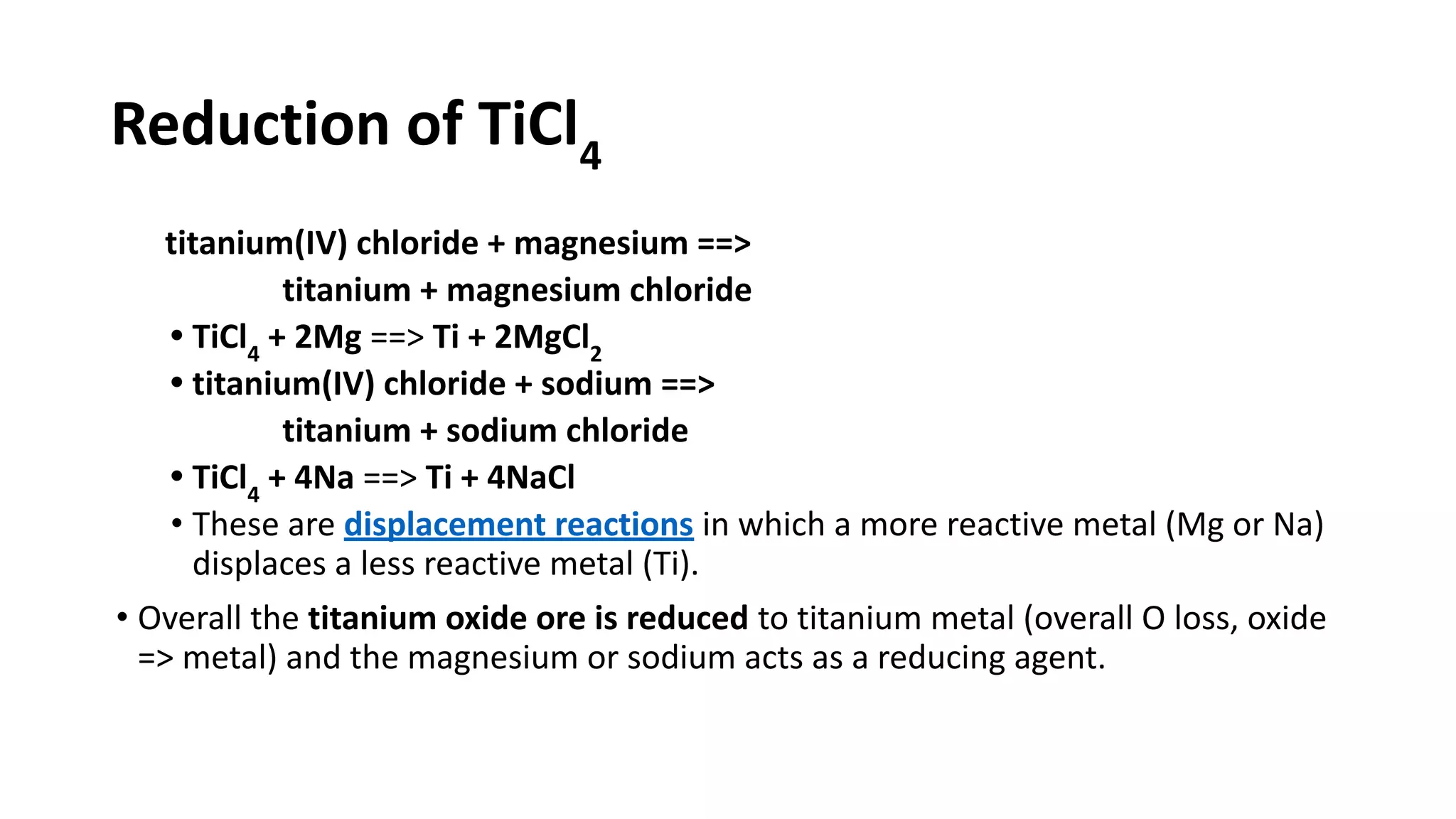
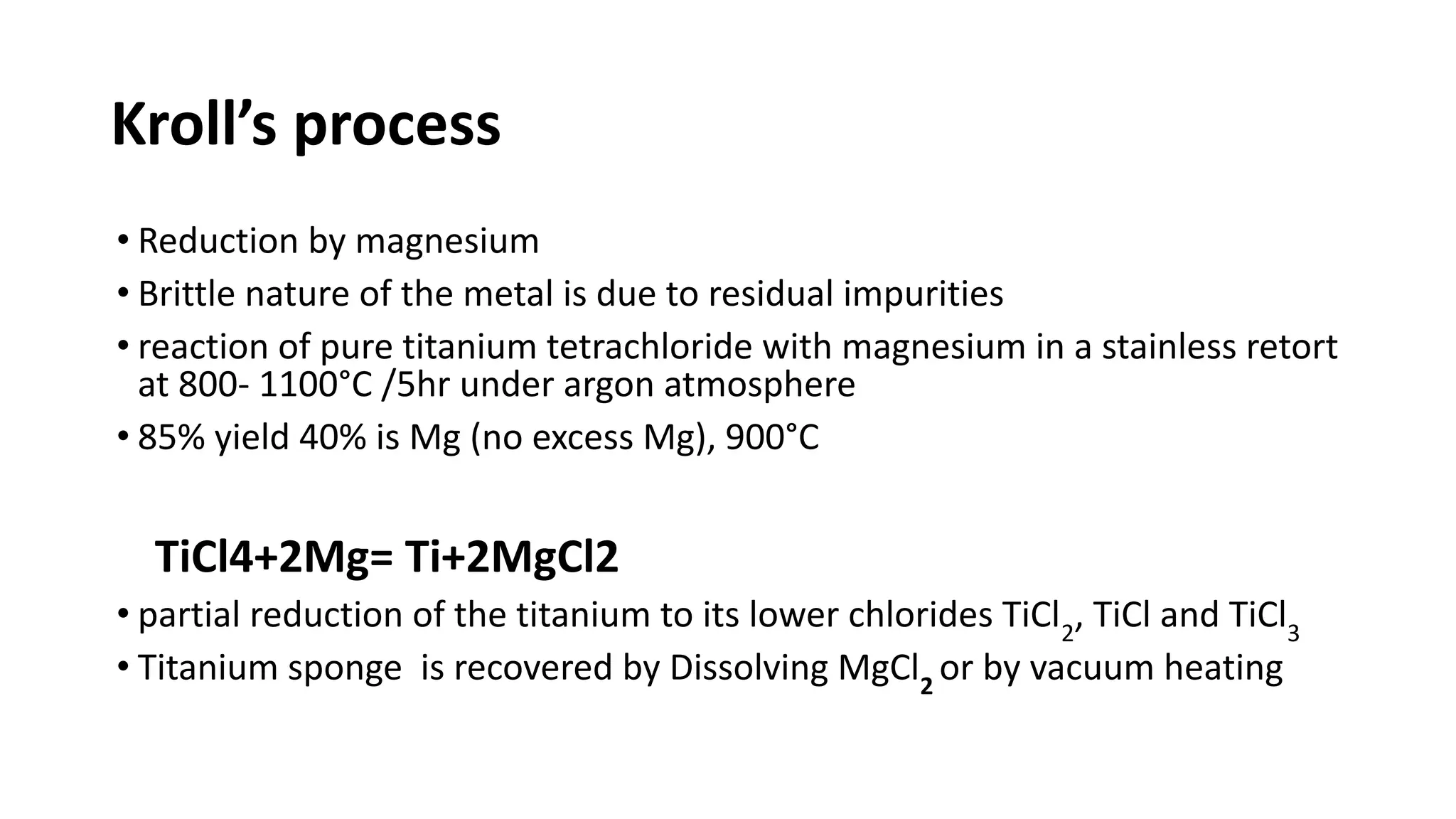
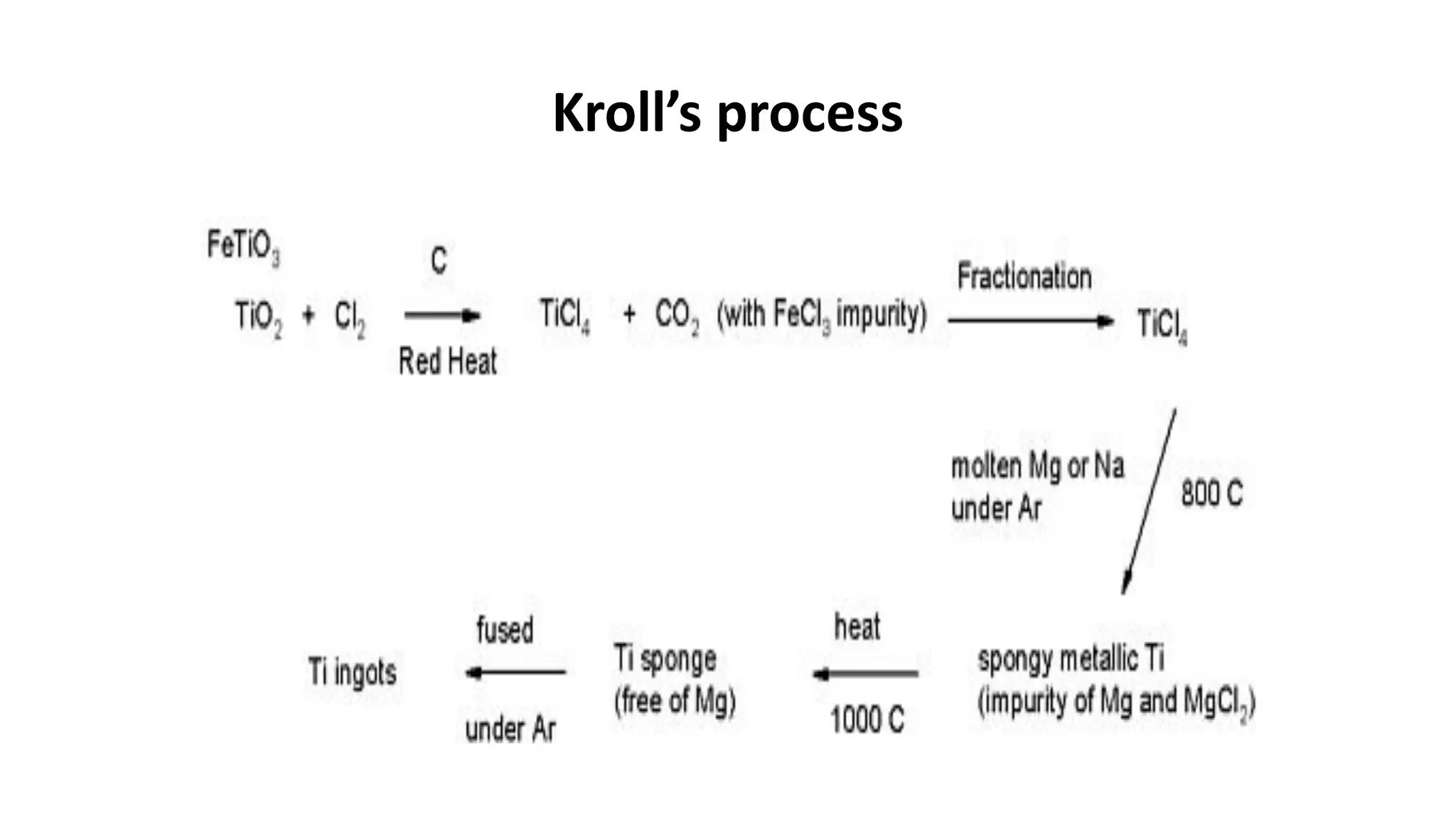
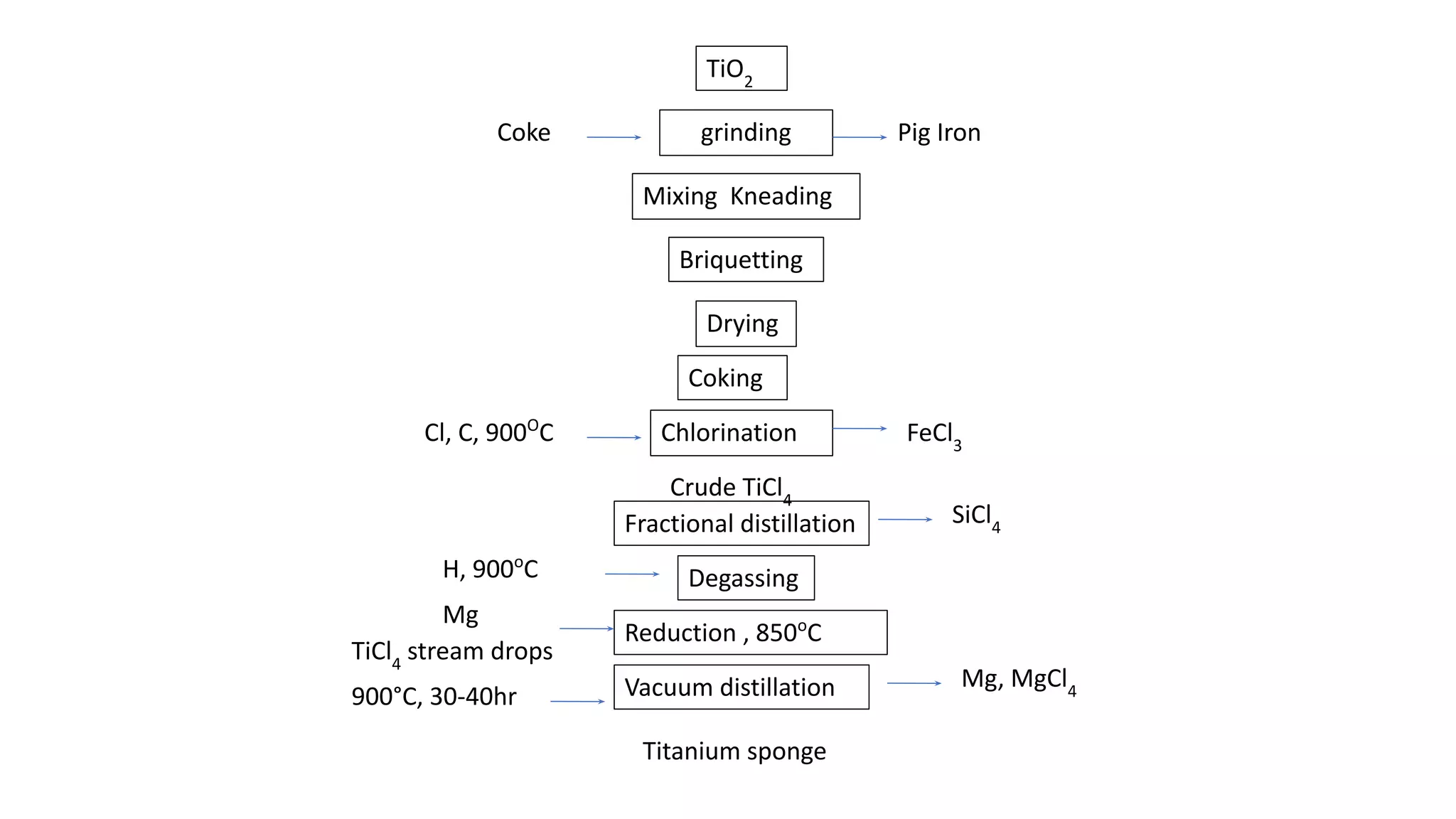
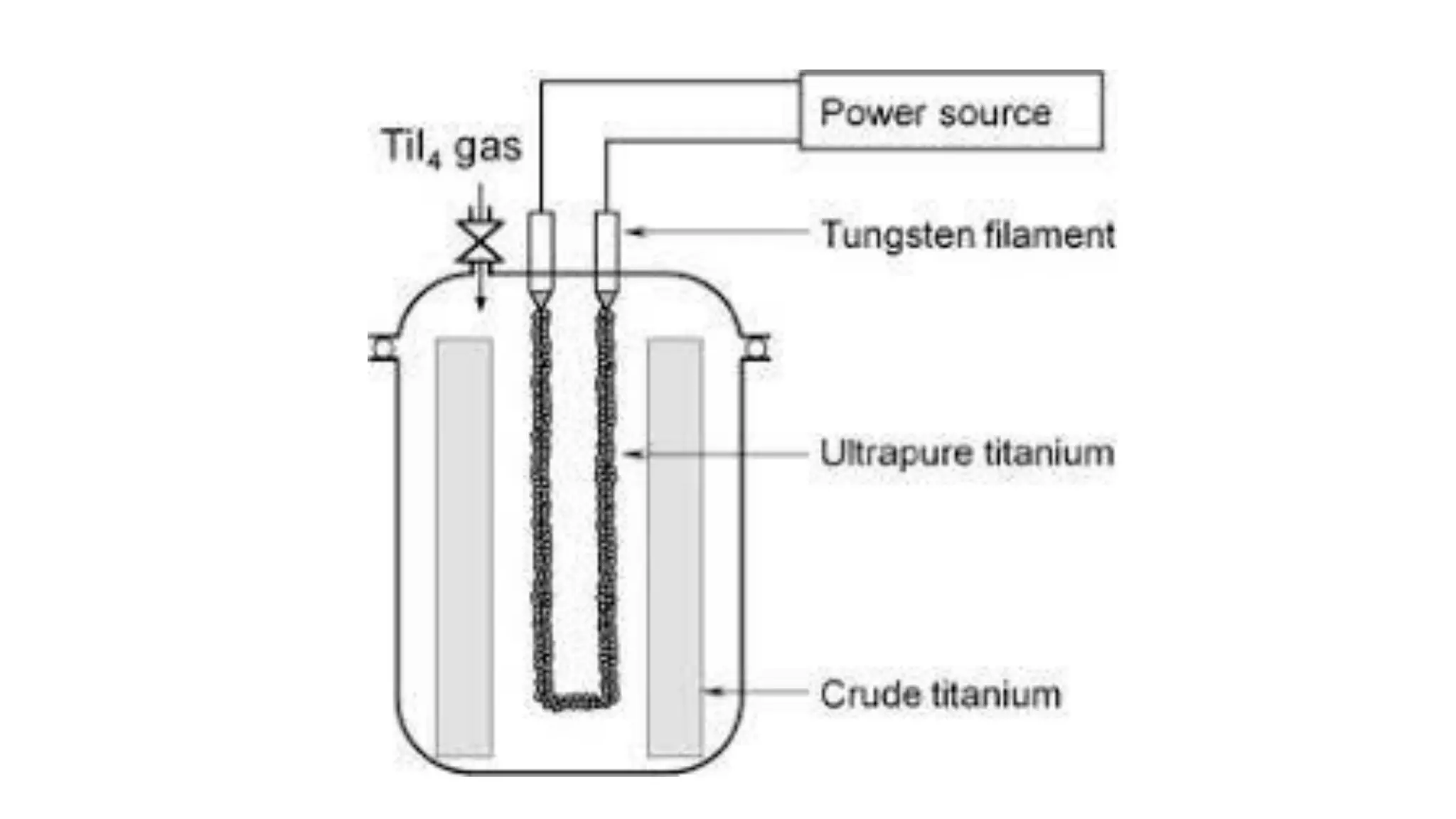
The document discusses the extraction process of titanium from its common mineral sources such as rutile and ilmenite. It involves upgrading the titanium content of ilmenite through smelting or acid leaching. Titanium dioxide is extracted from these sources and converted to titanium tetrachloride via chlorination. The titanium tetrachloride is then reduced to titanium metal using magnesium or sodium in the Kroll process. The properties and applications of titanium in aerospace, chemical and nuclear industries are also mentioned.