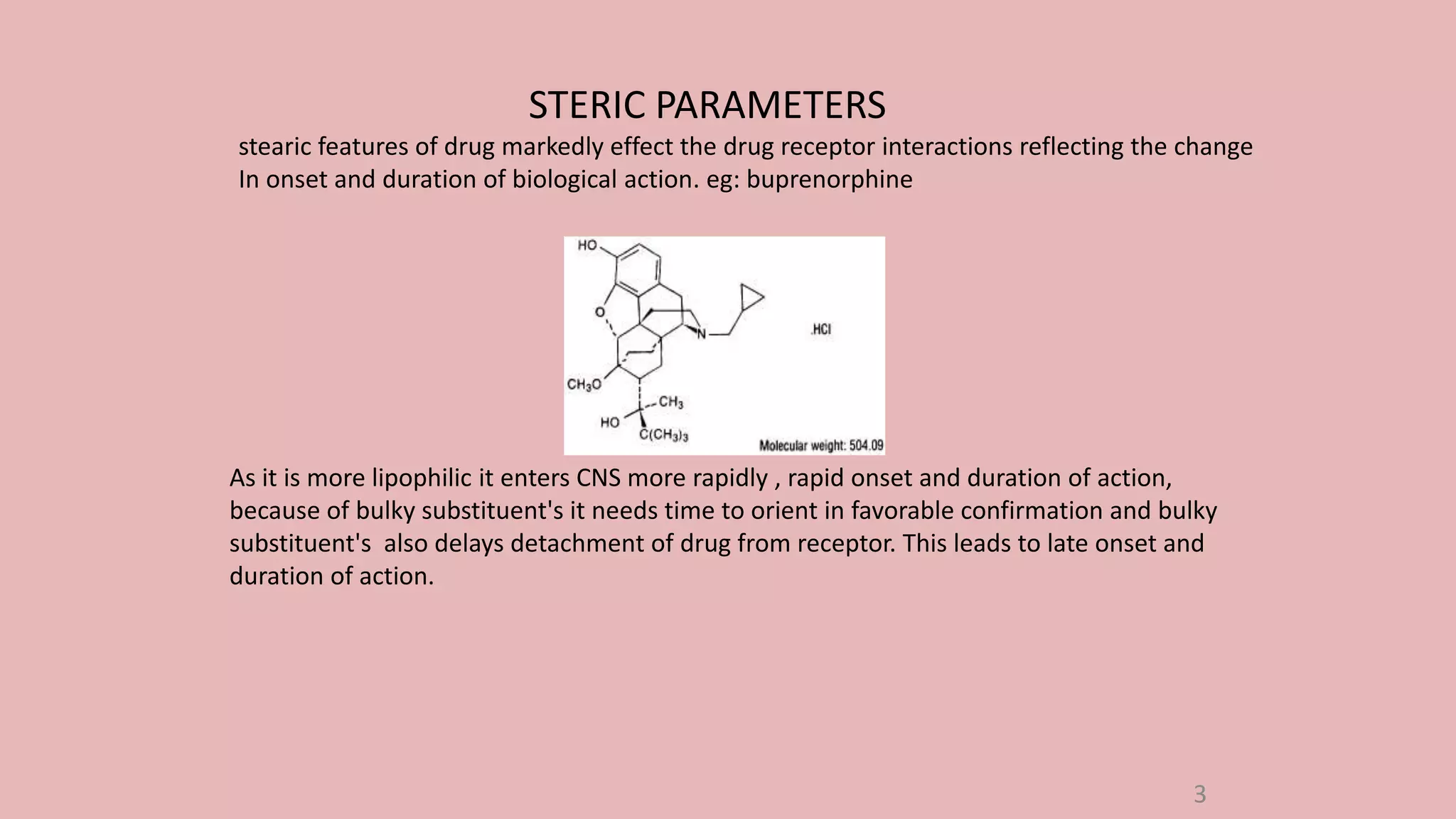
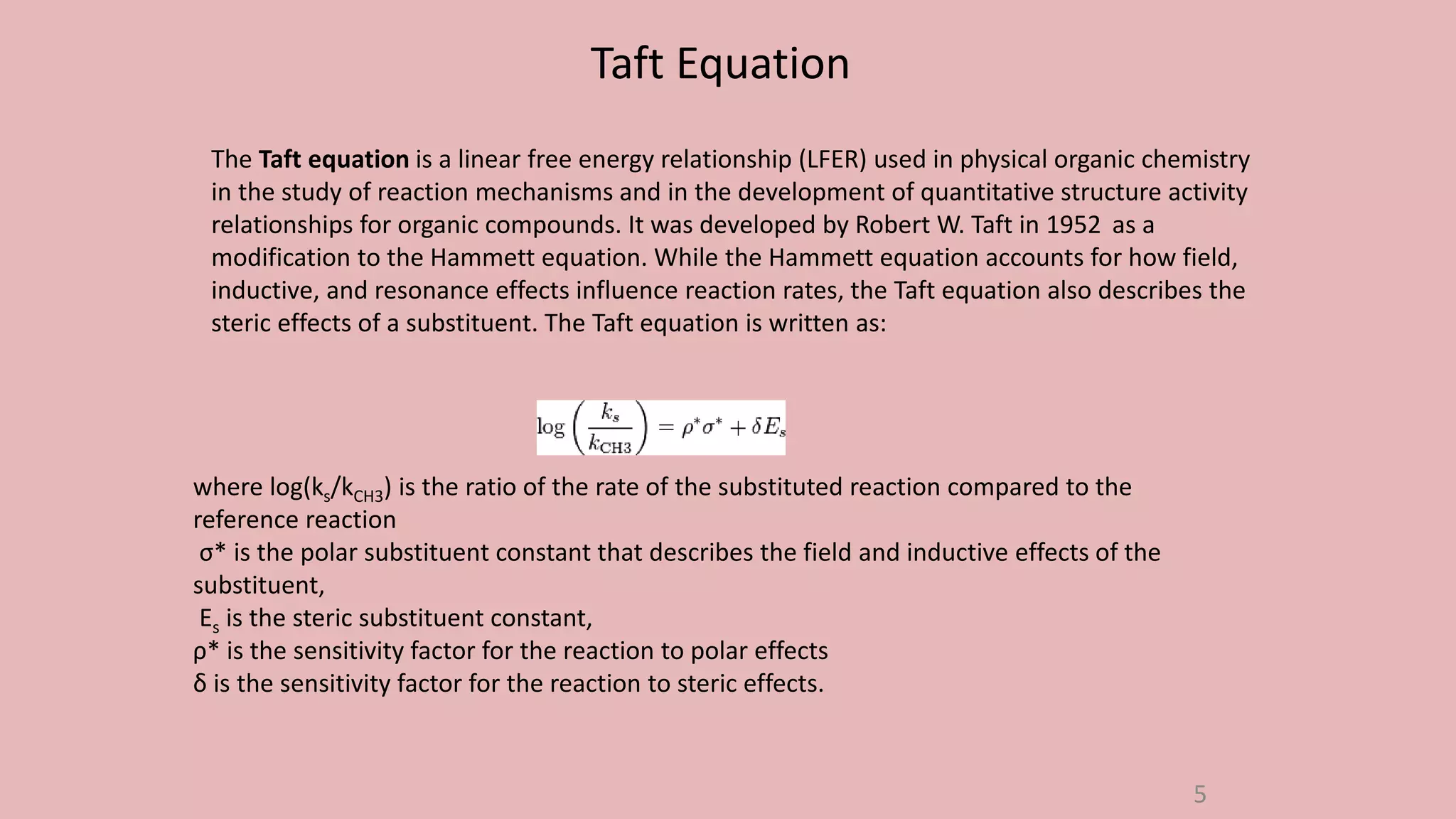
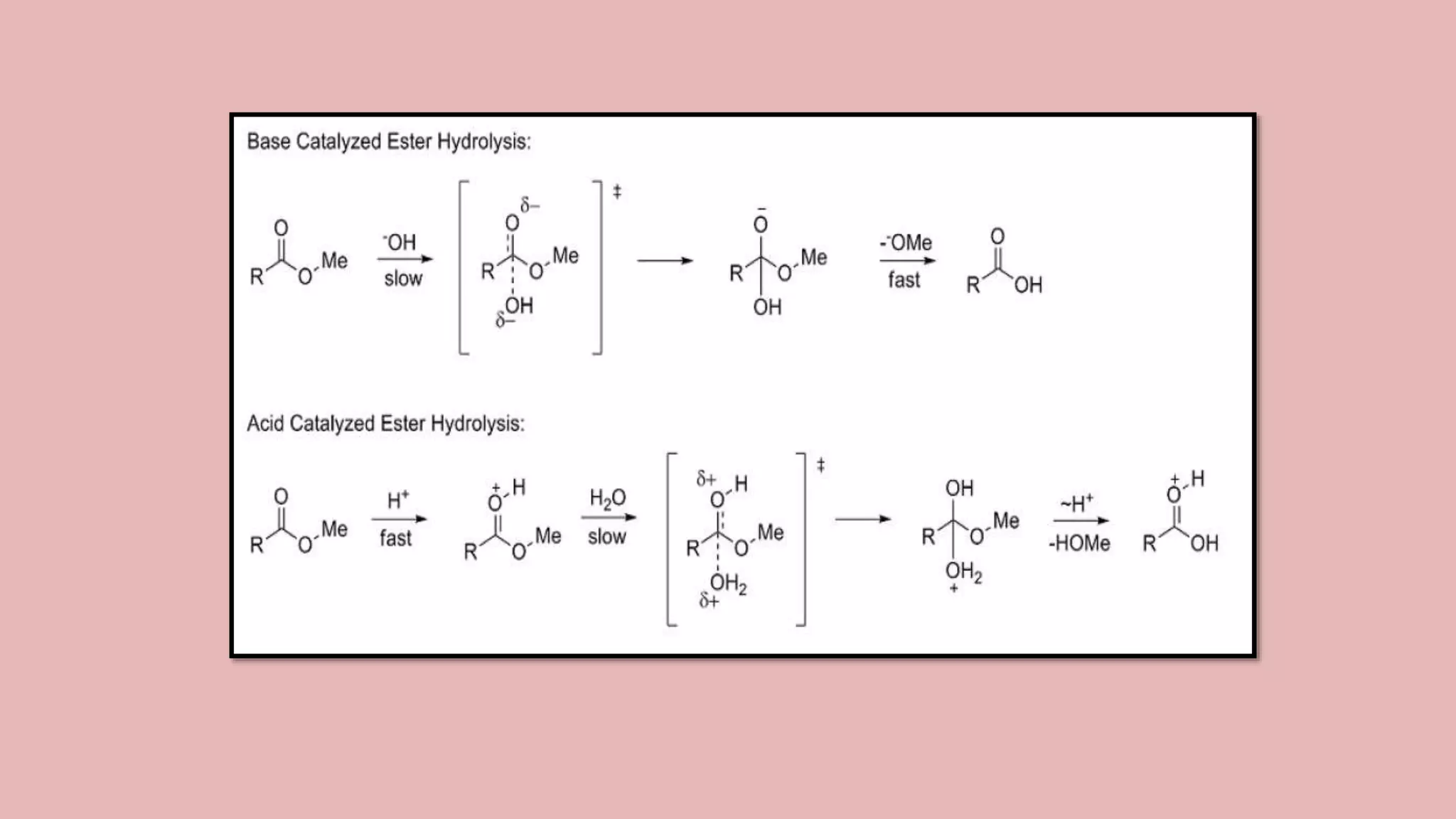
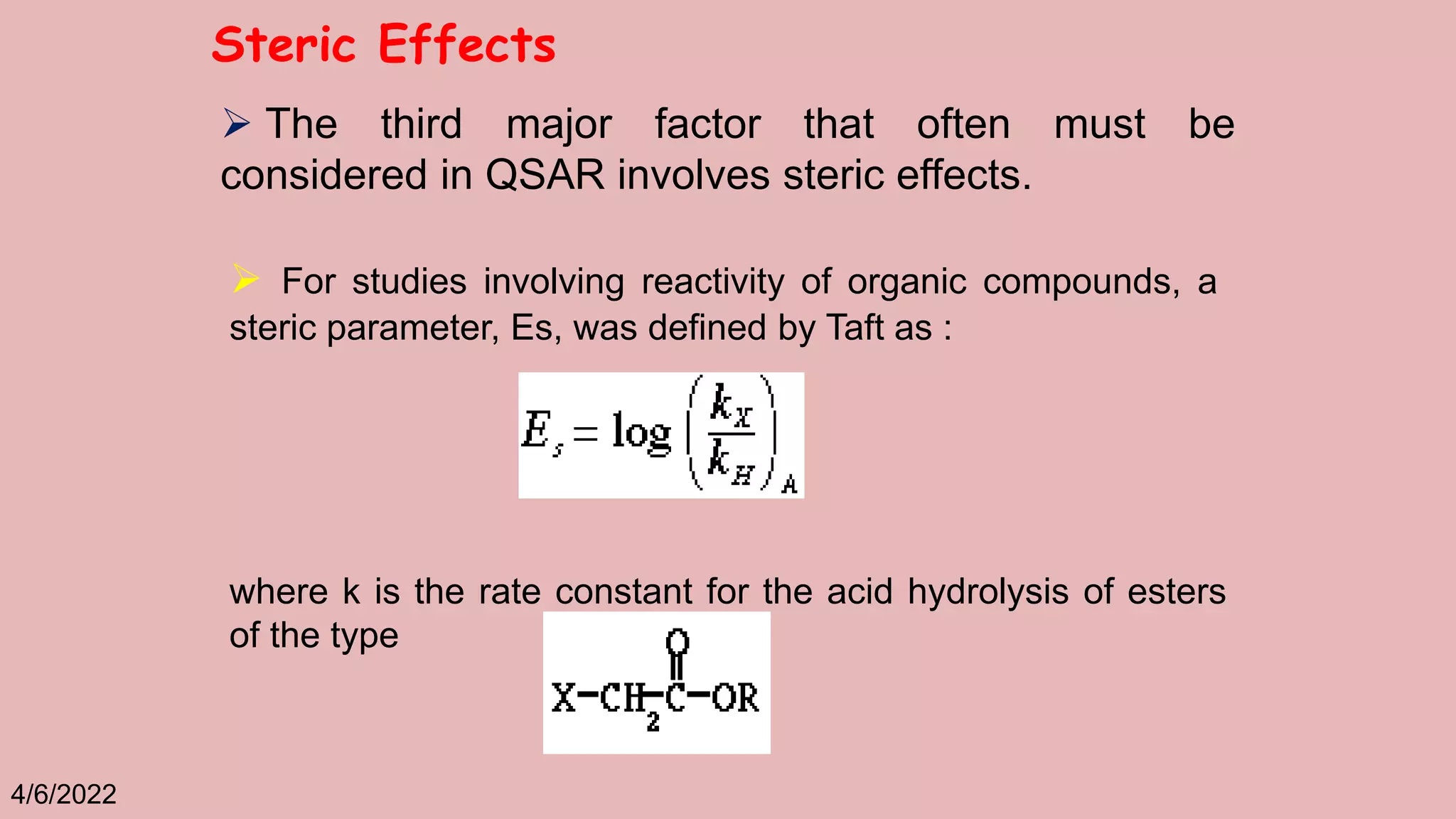
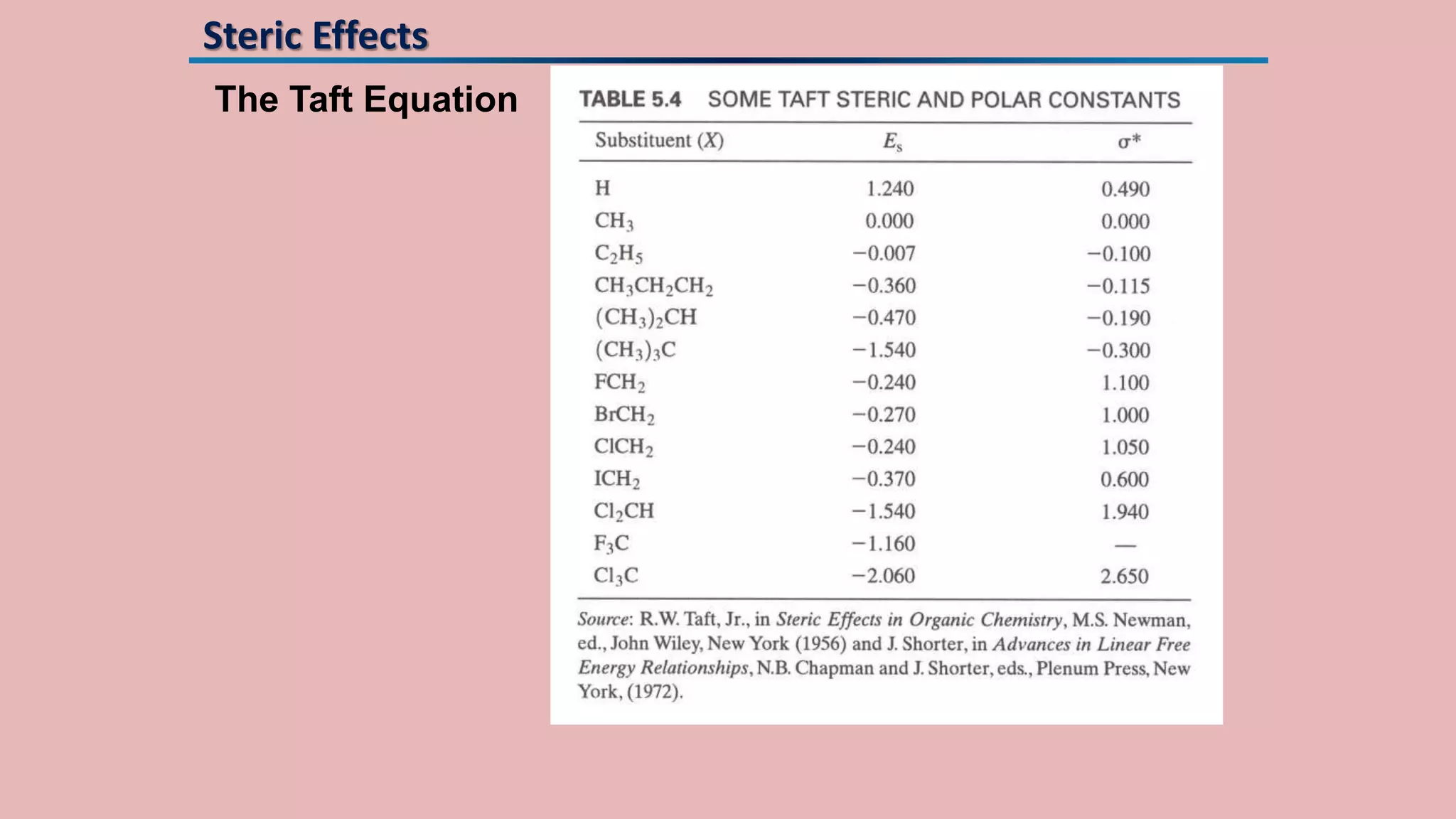
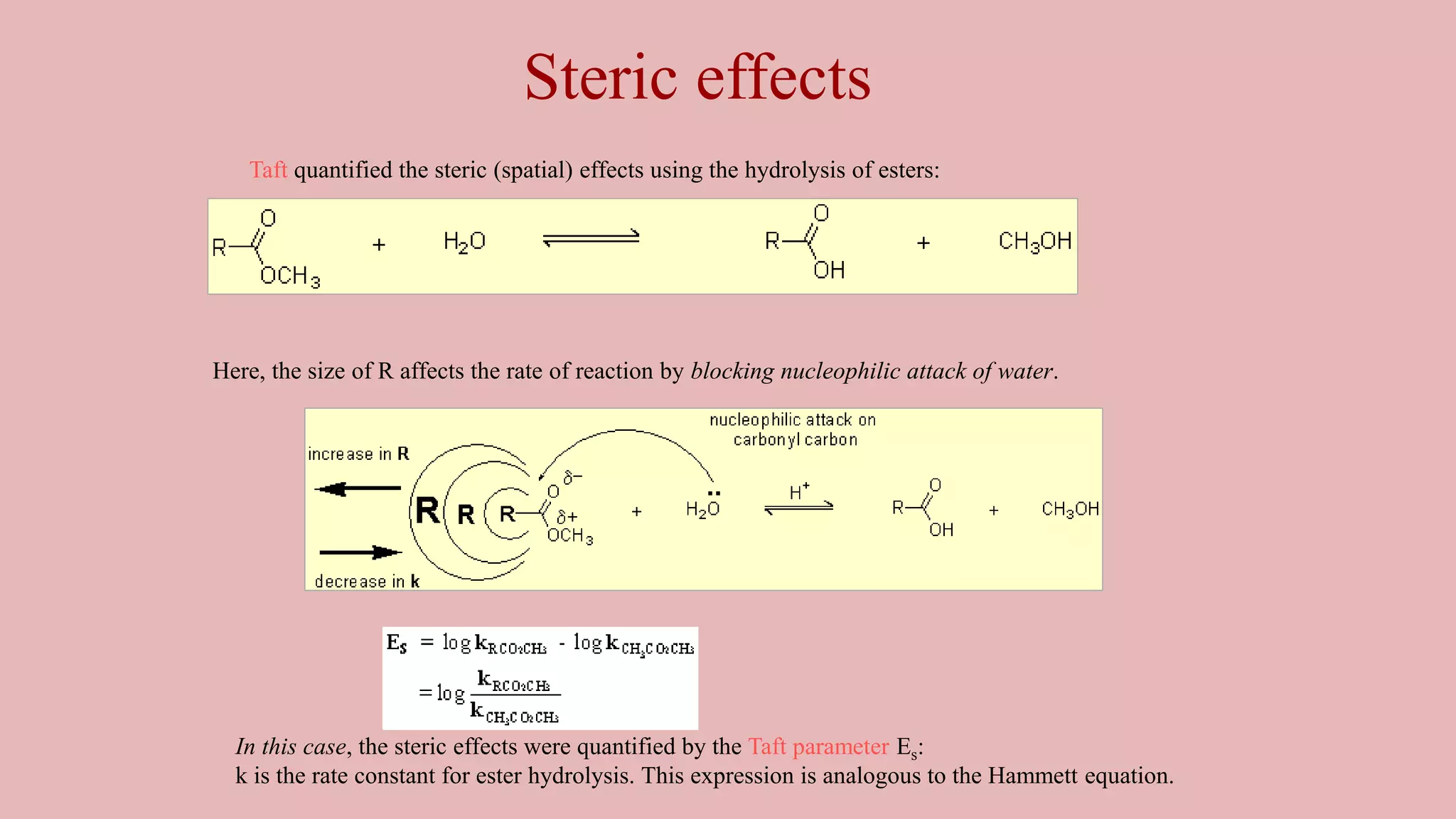
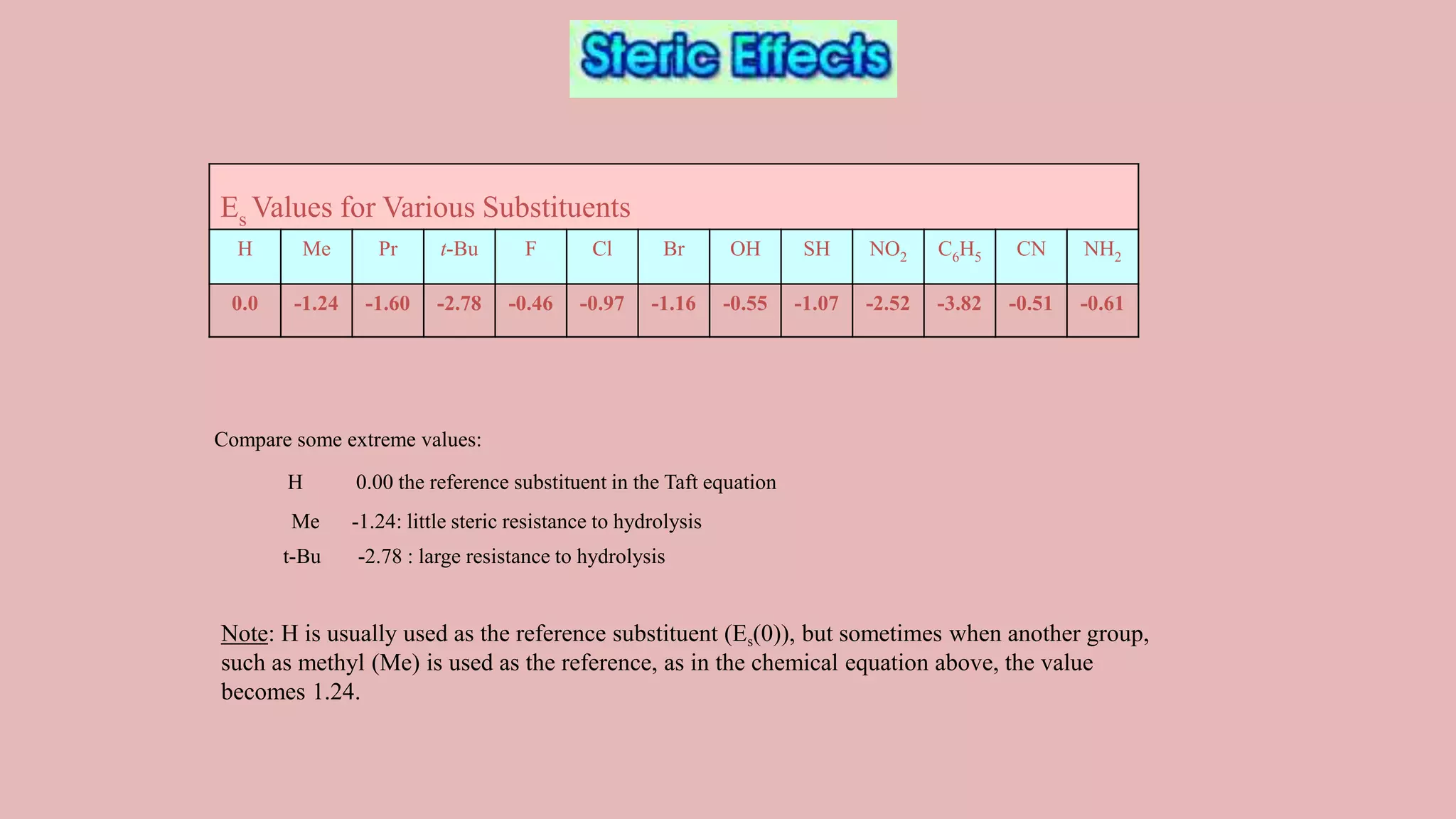
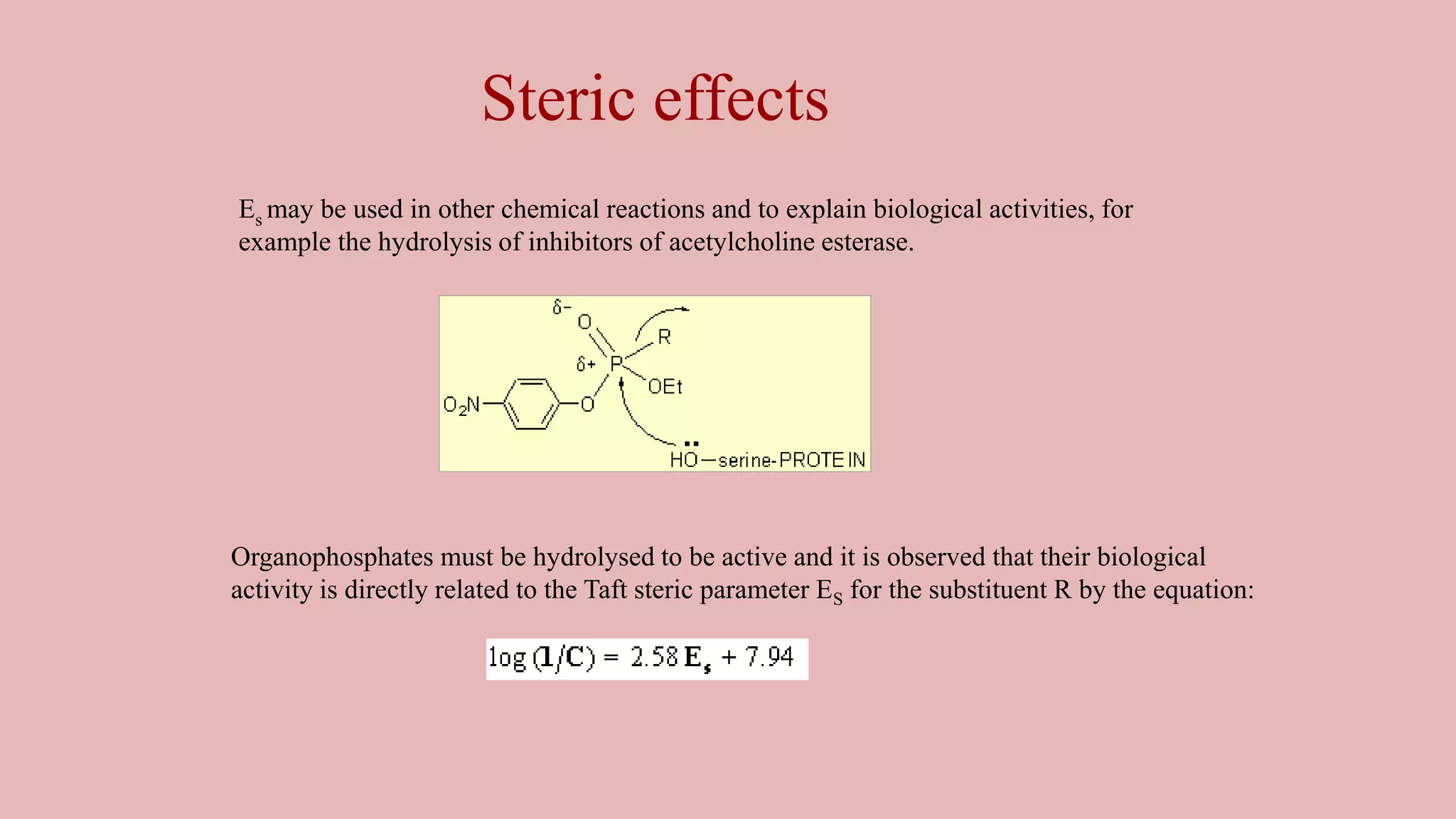
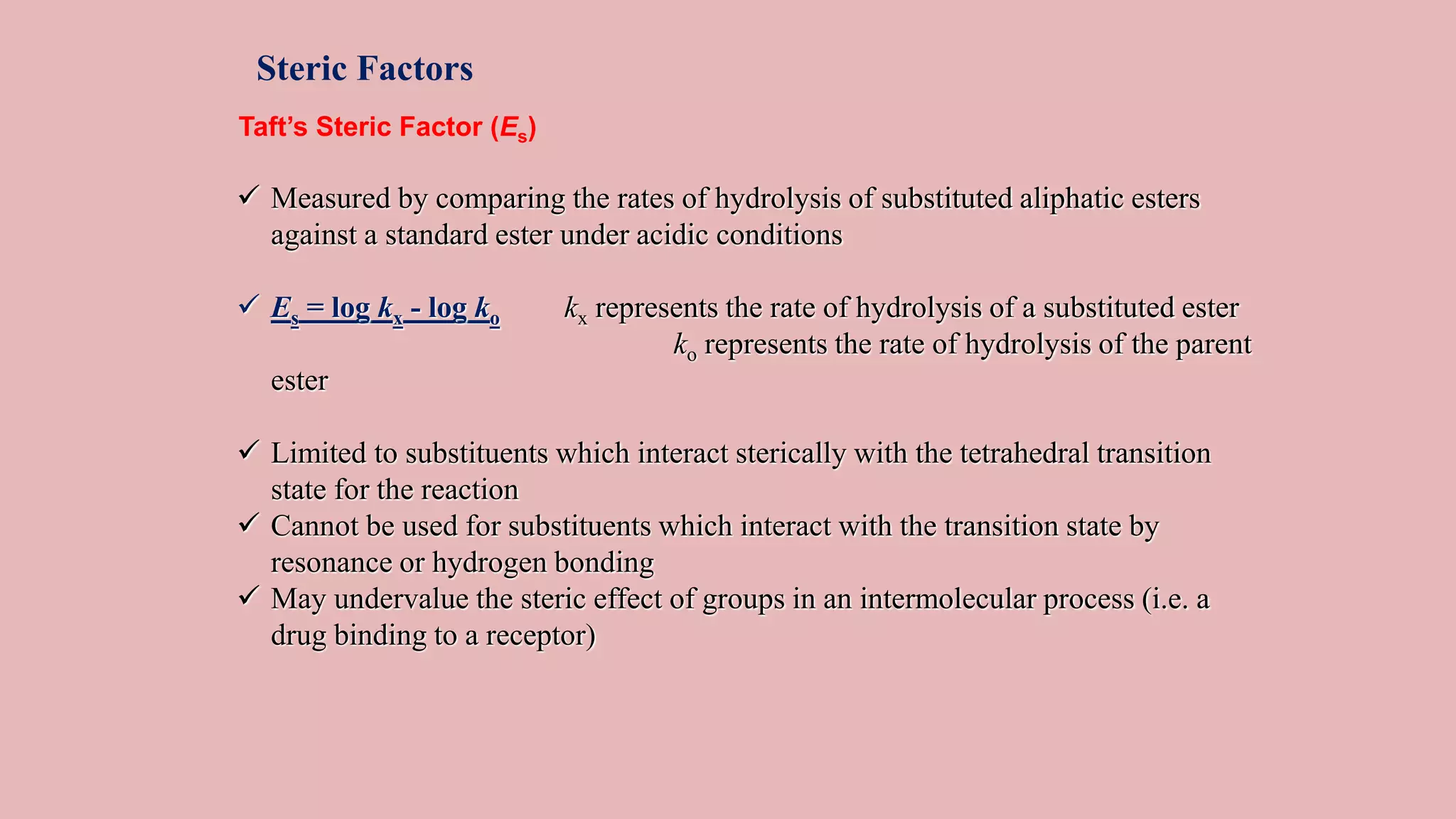
The document discusses Taft's steric factor (Es) as a way to quantify steric effects in organic compounds. Es is based on rate constants of ester hydrolysis reactions and accounts for how bulky substituents affect reaction rates by blocking nucleophilic attack. The Taft equation combines Es with other substituent constants to model steric and electronic effects. Examples show Es is more negative for bulkier groups like t-Bu, indicating their stronger steric hindrance of hydrolysis. Es can be used to understand and predict steric influences on other chemical reactions and biological activities.