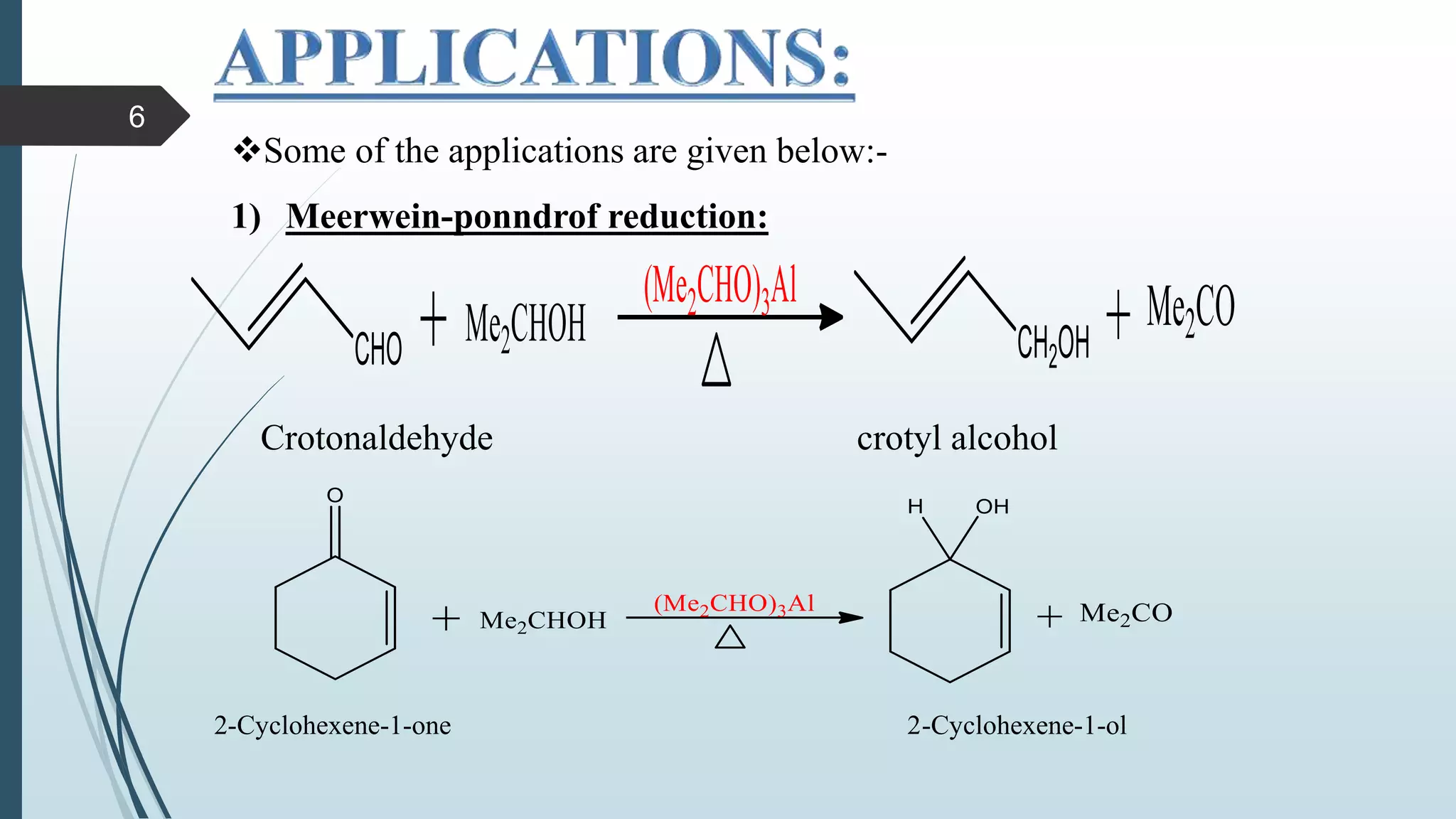
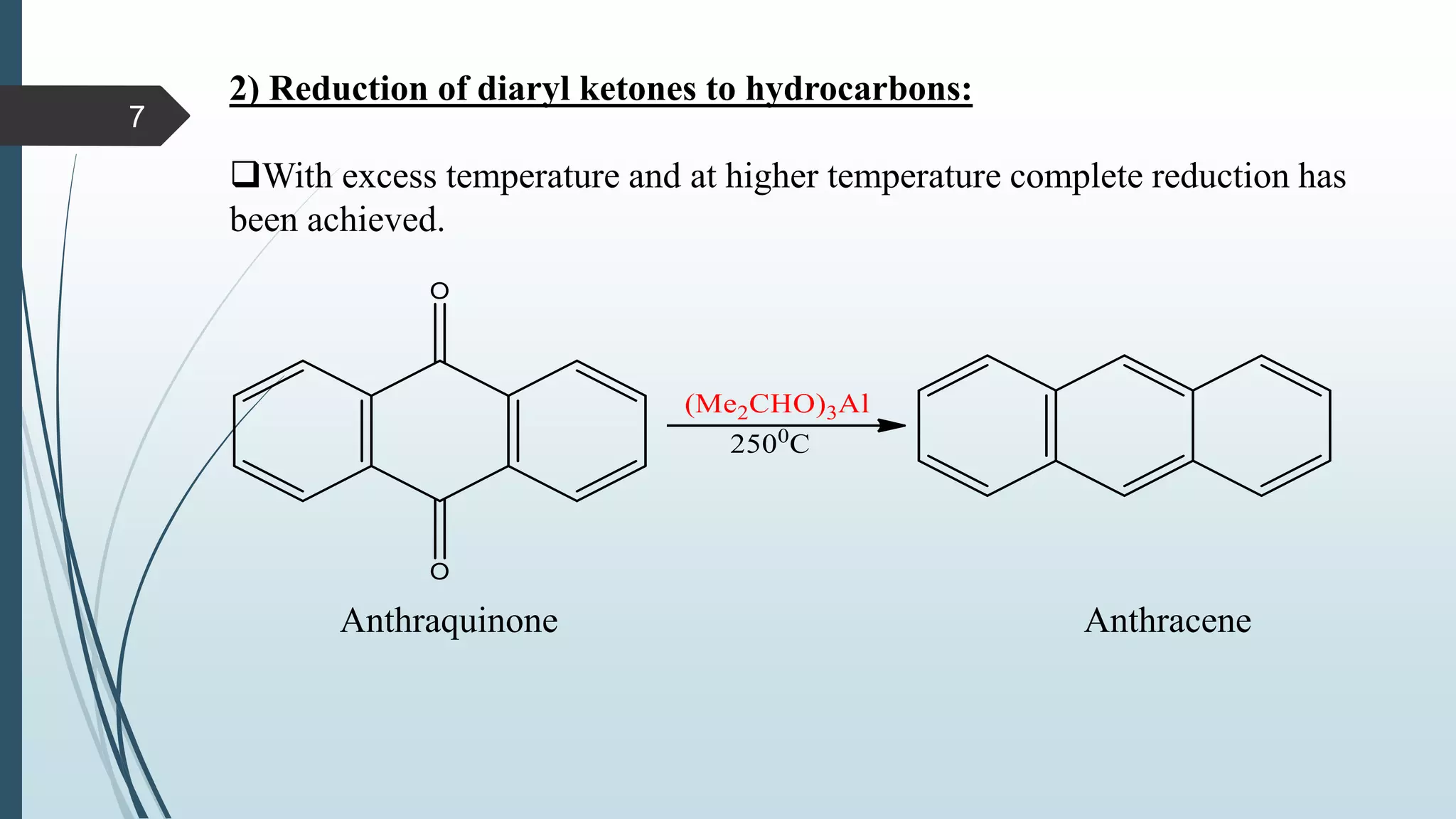
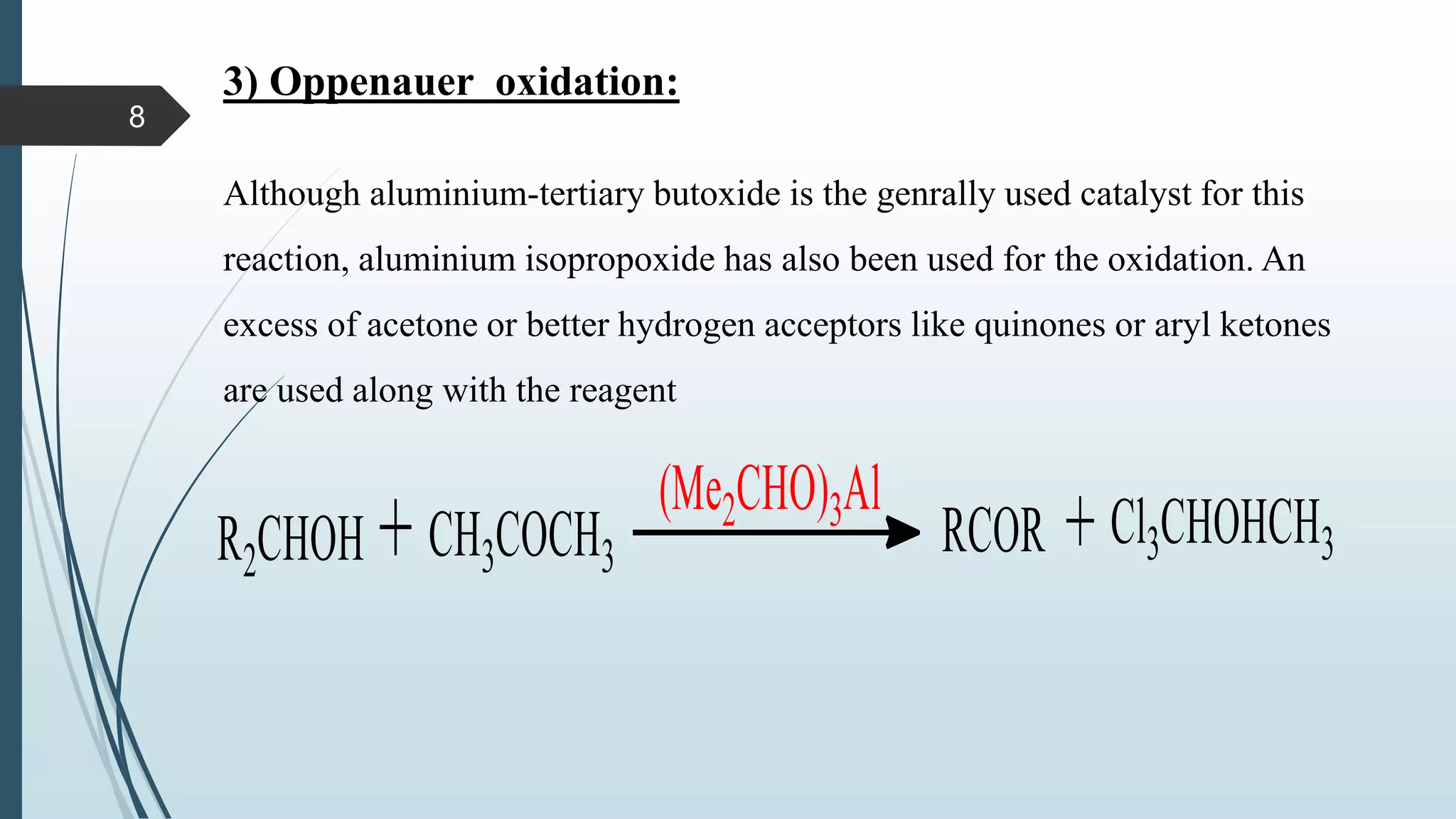
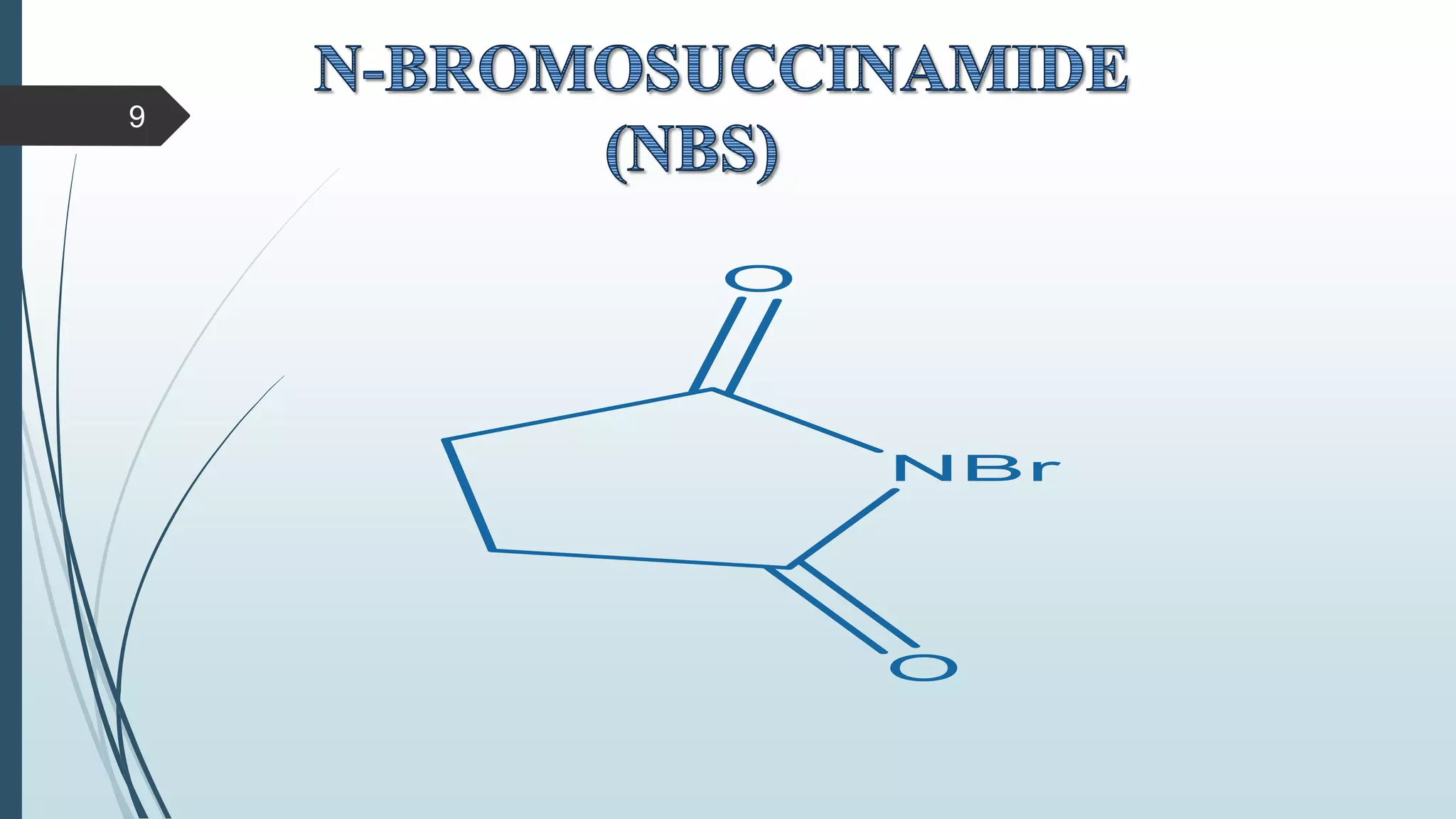
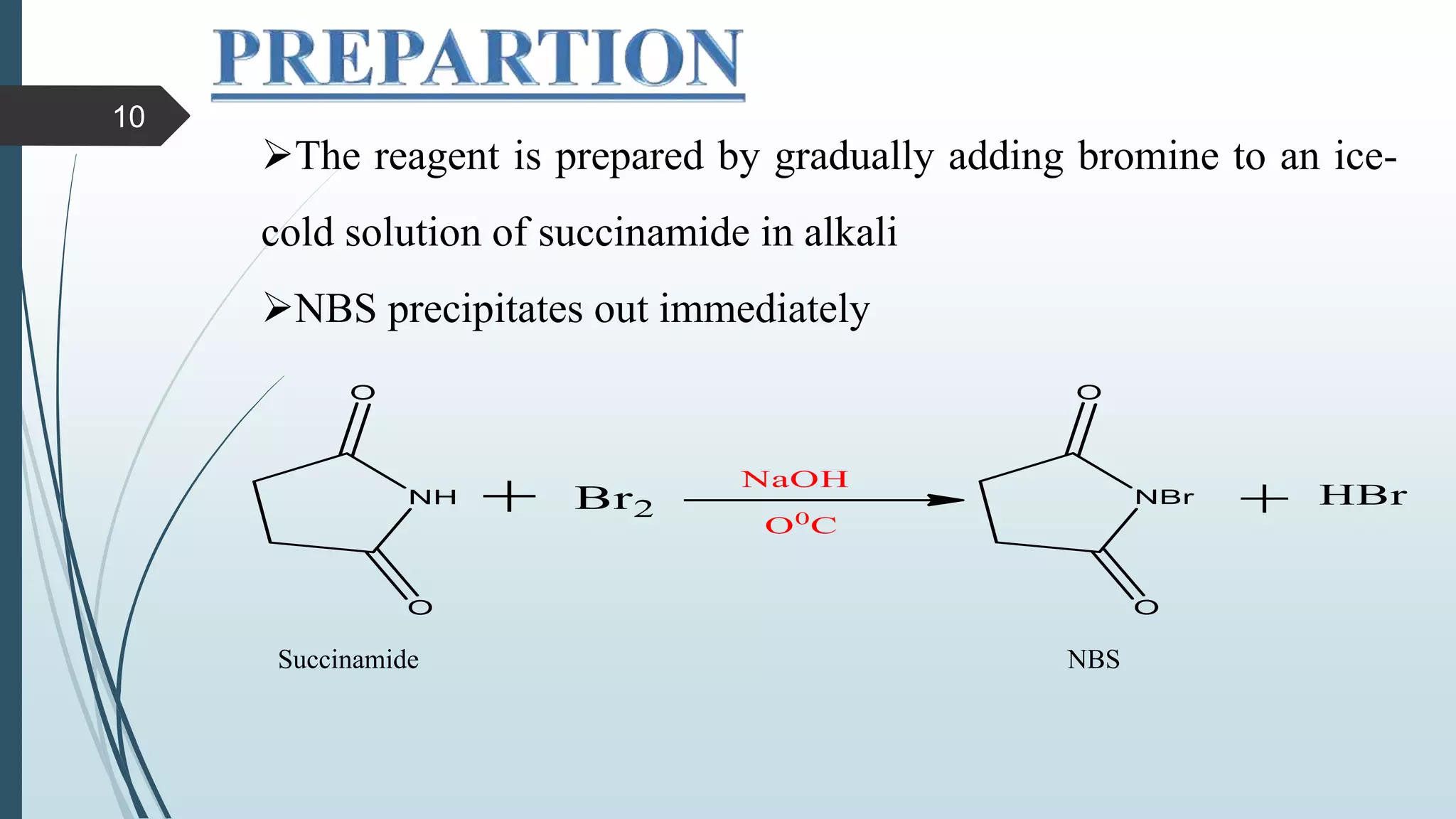
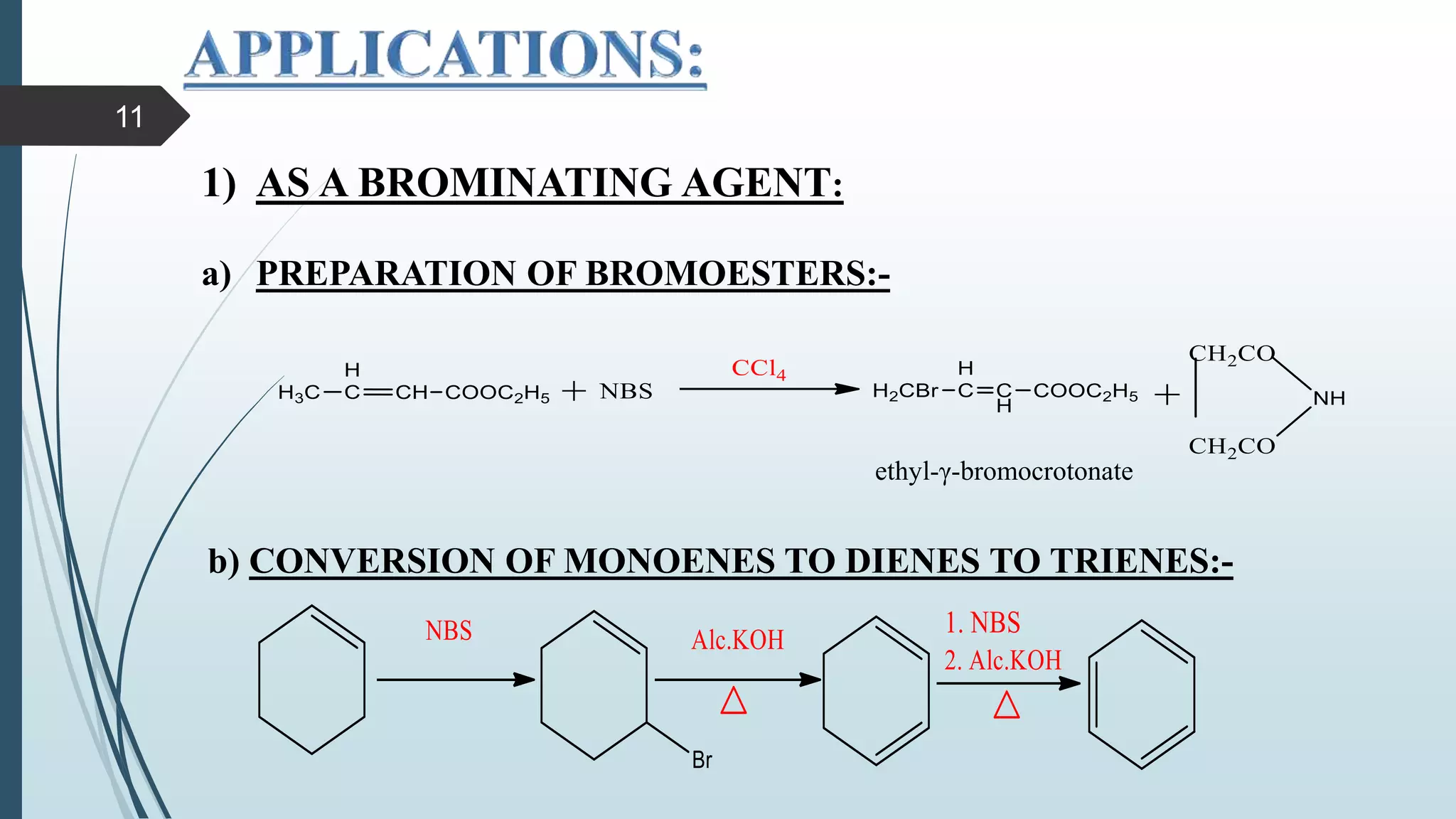
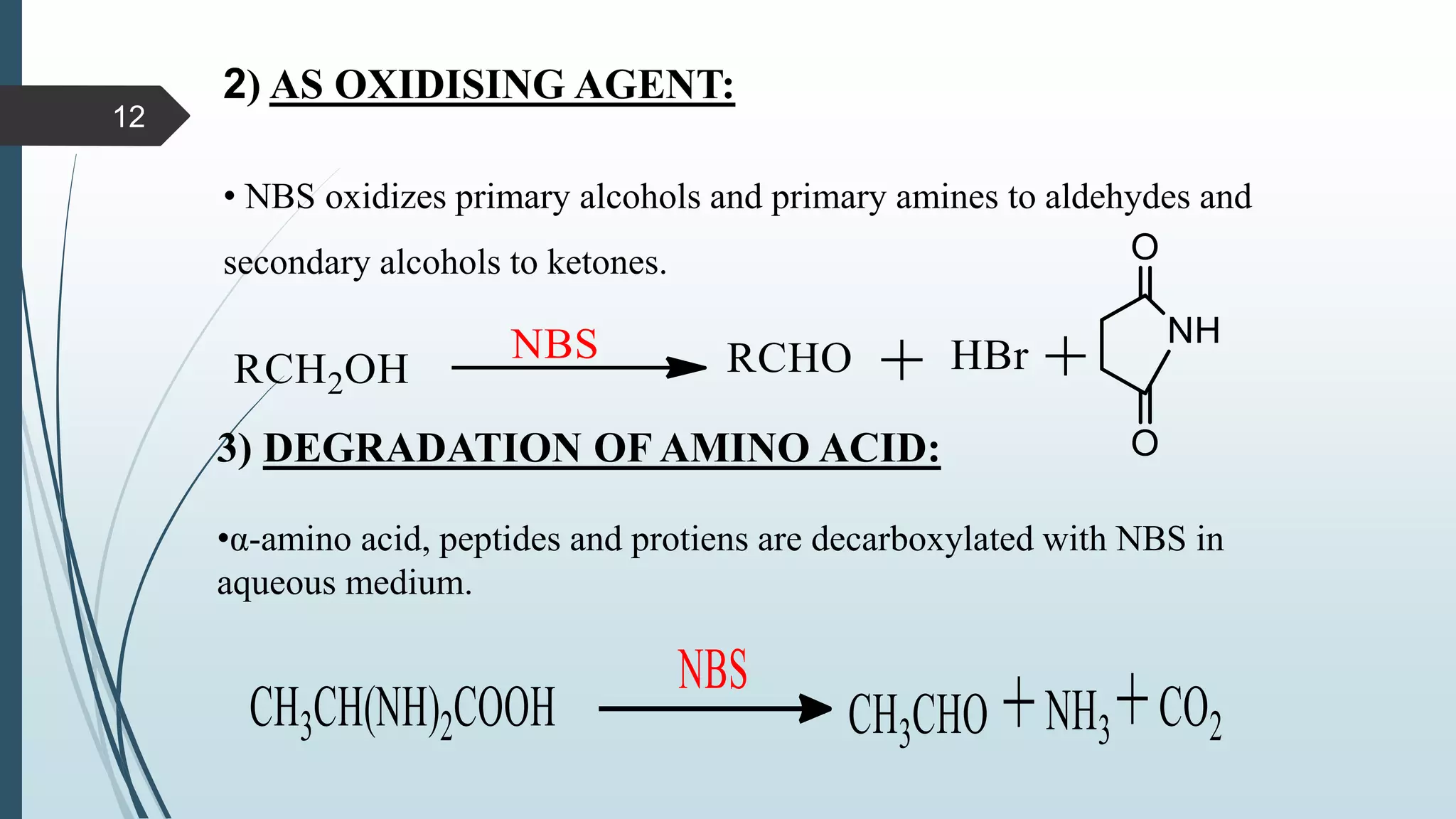
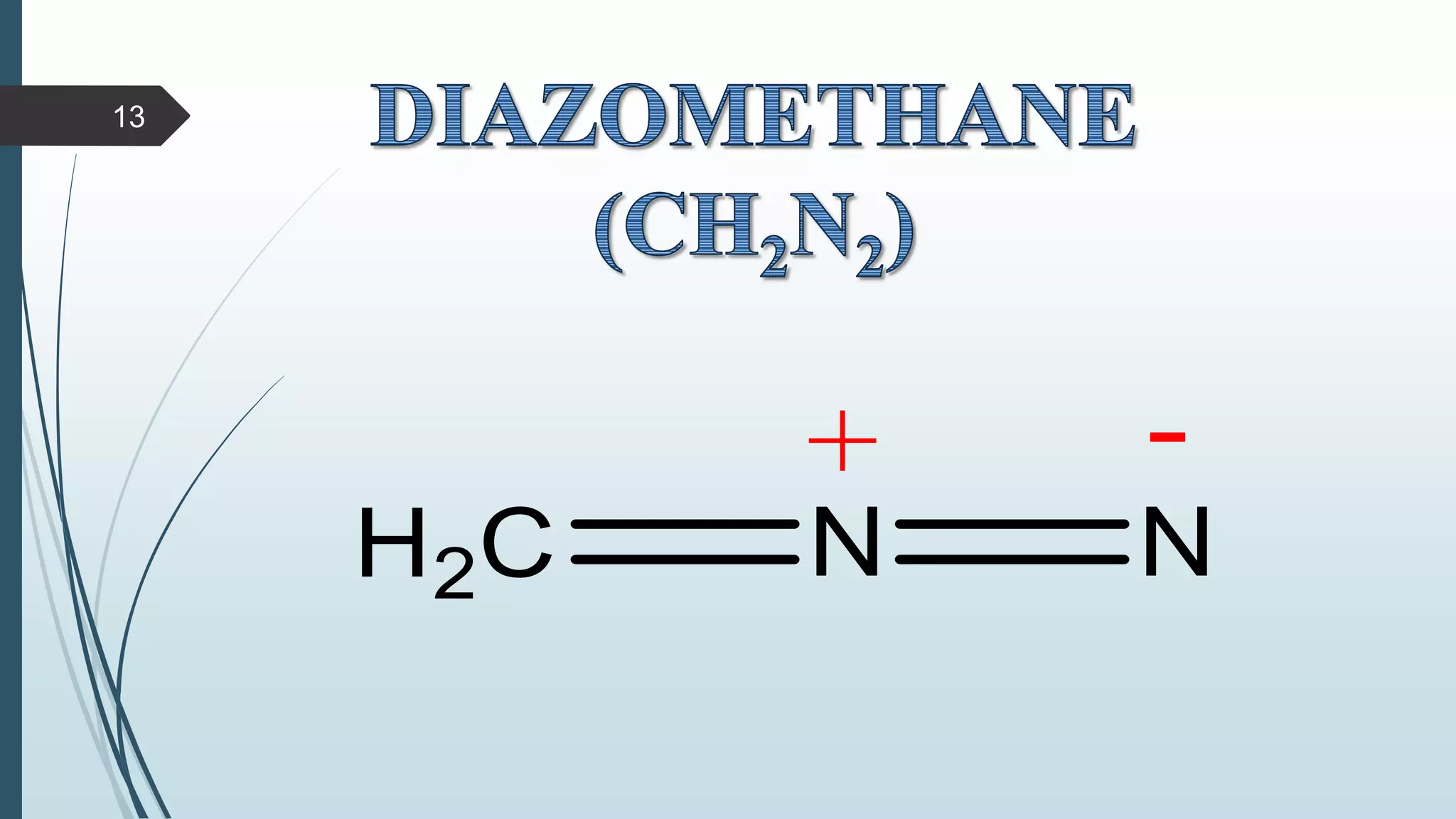
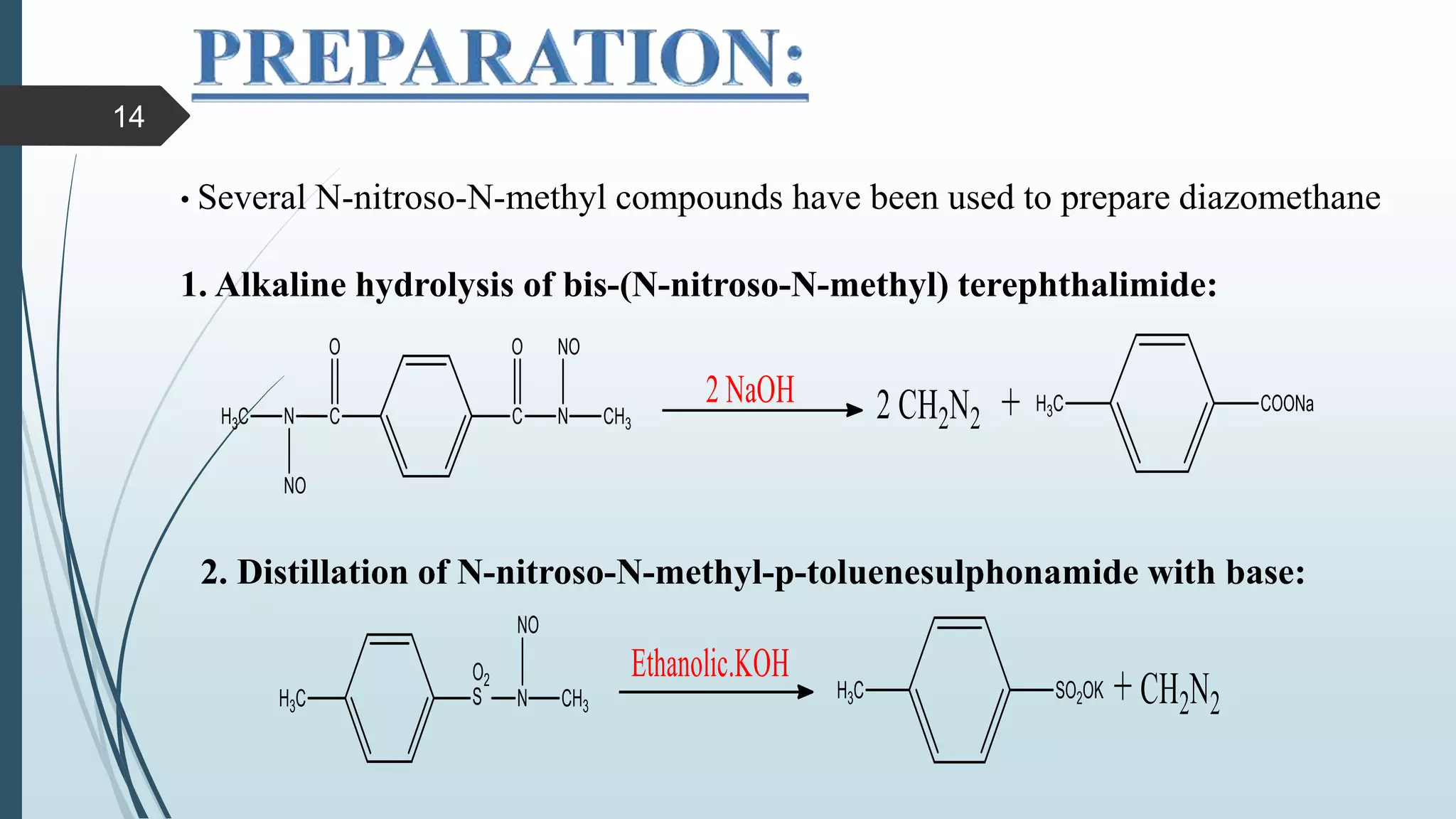
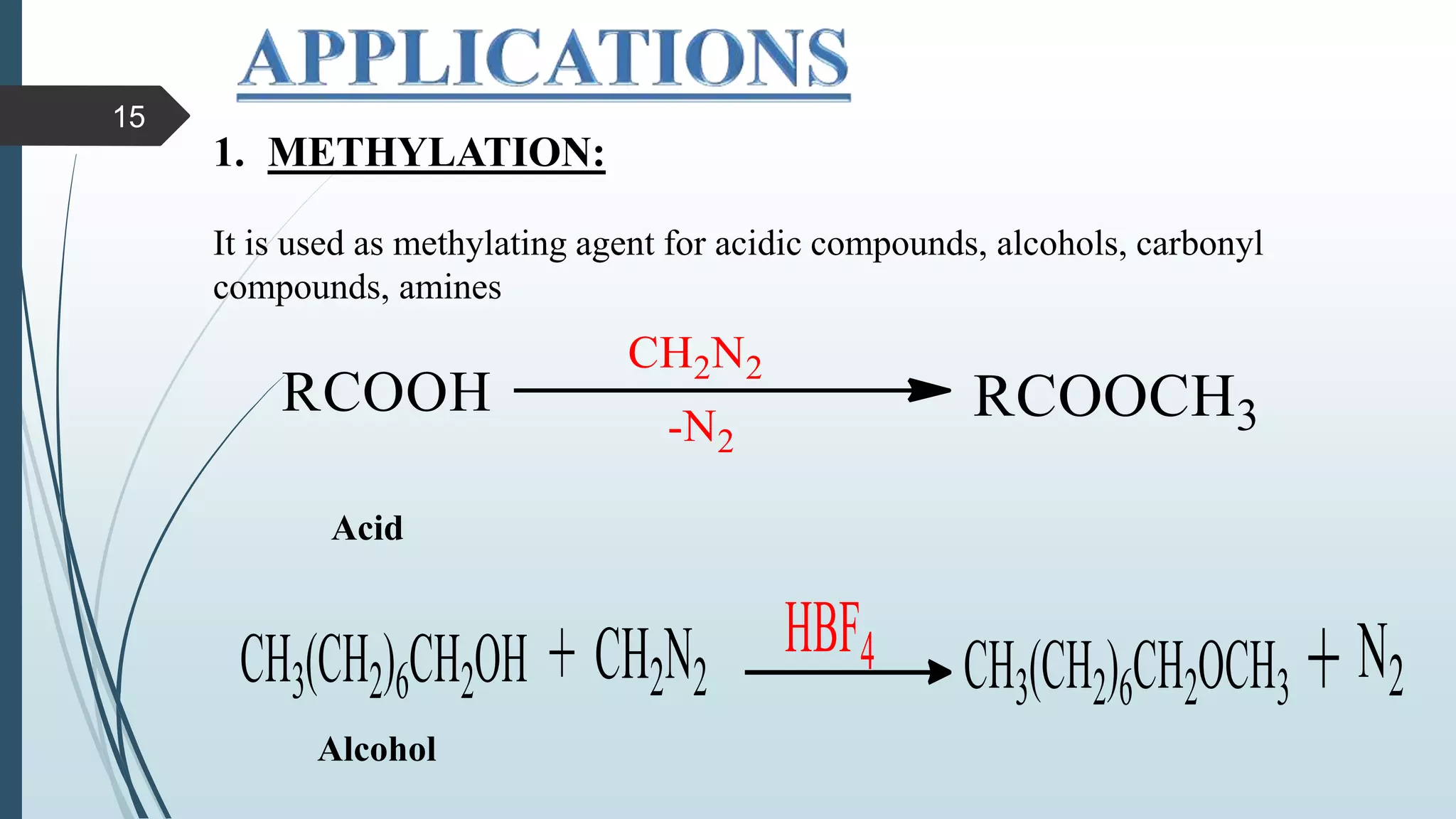
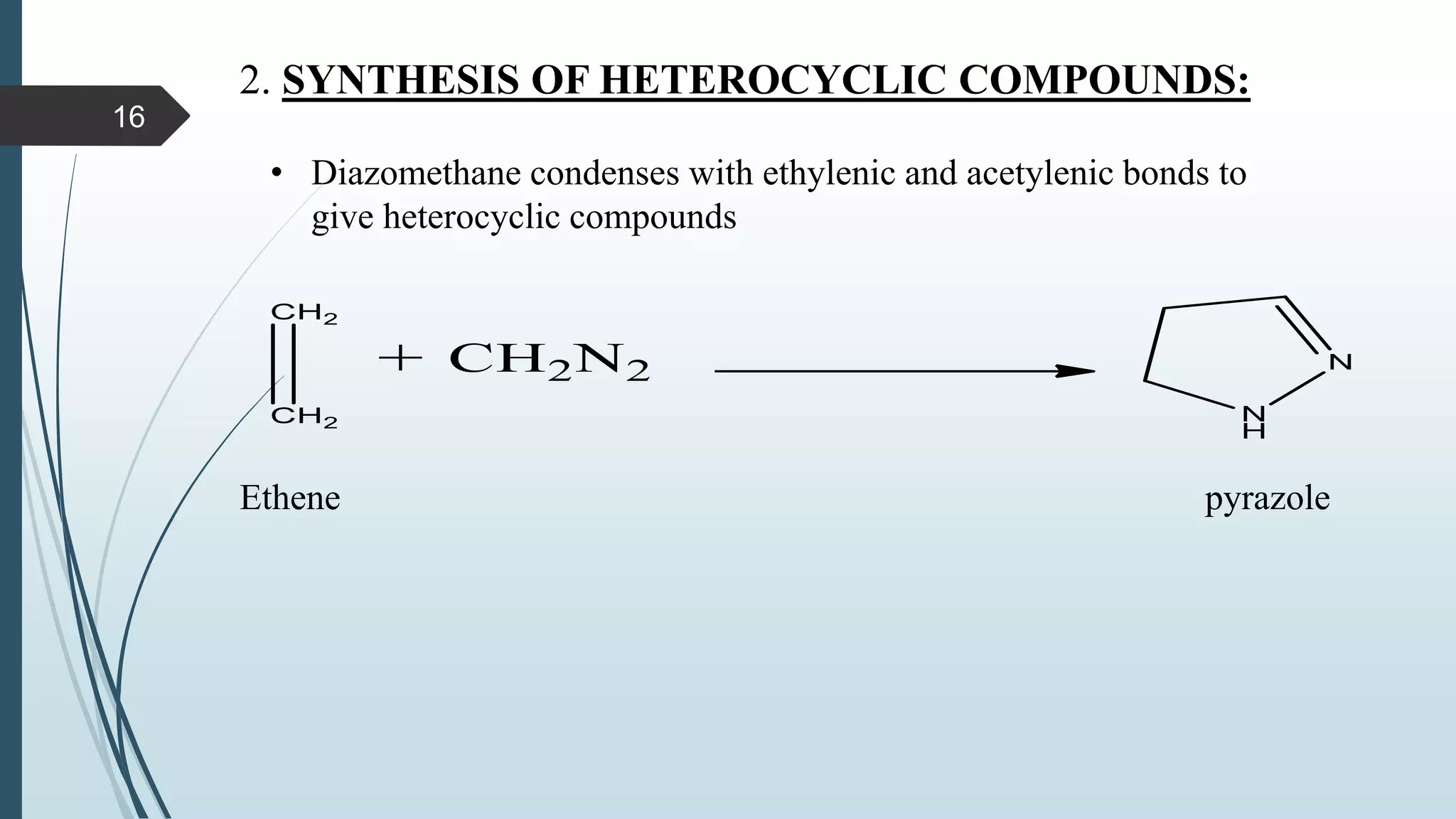
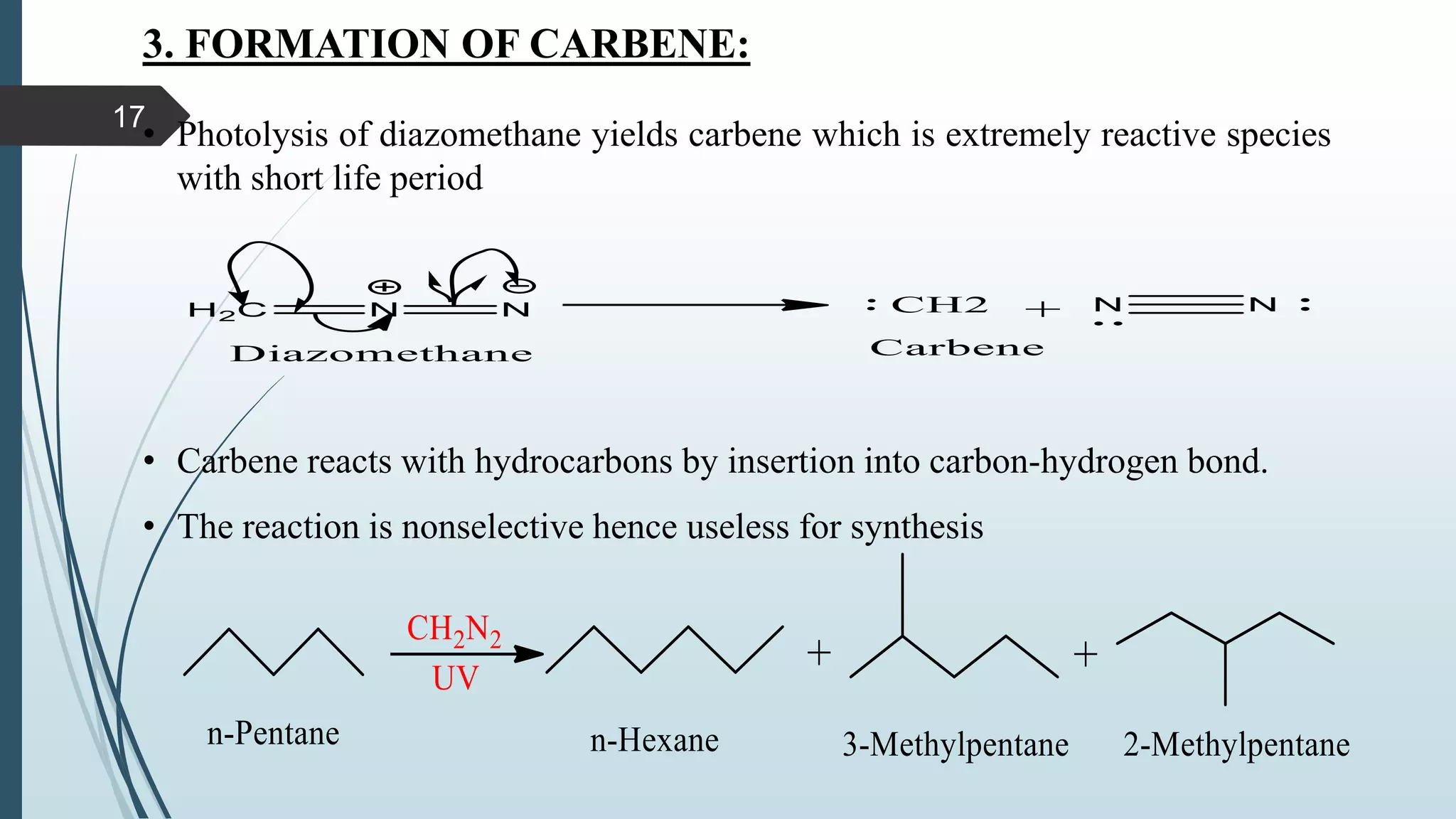
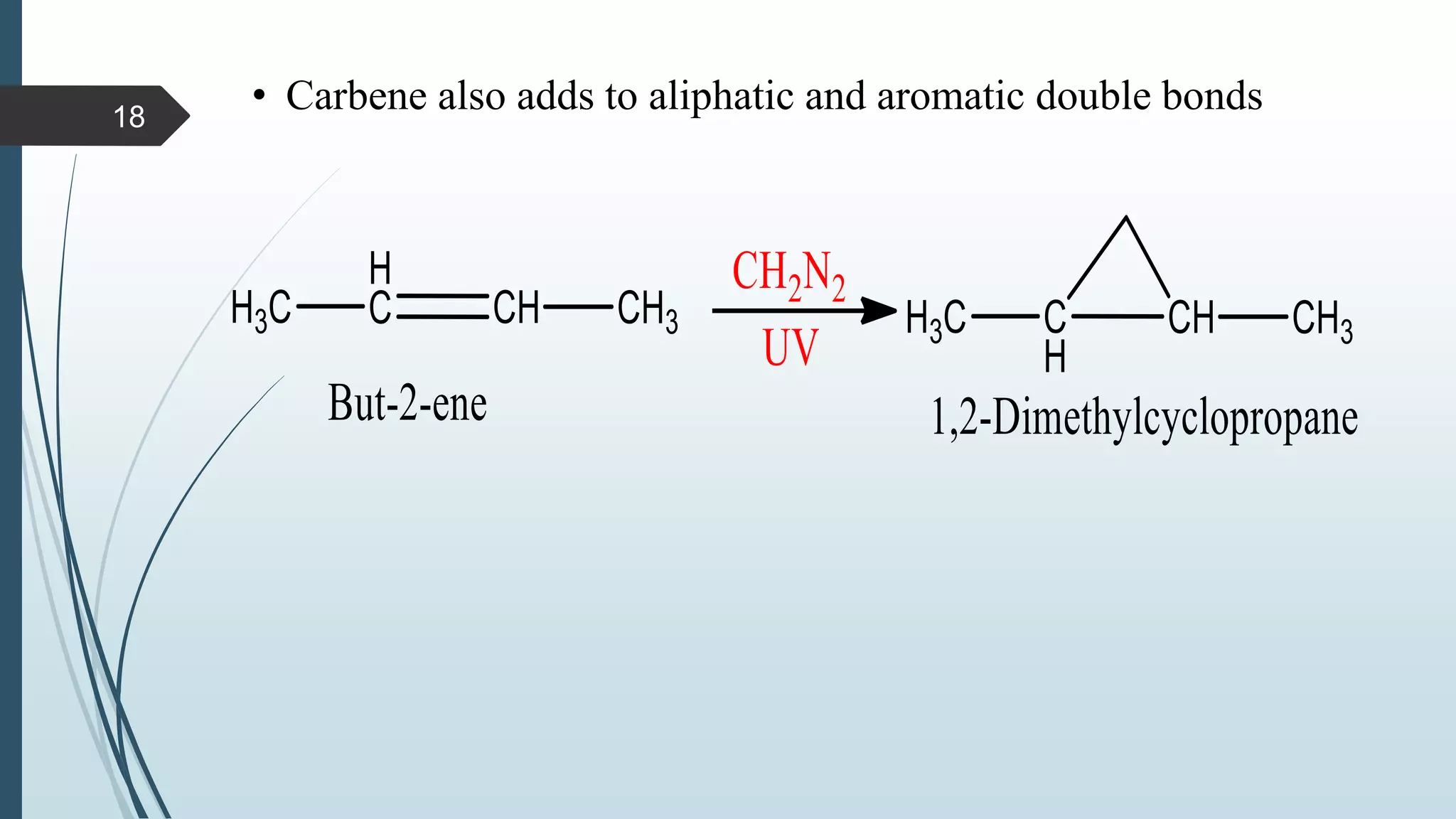
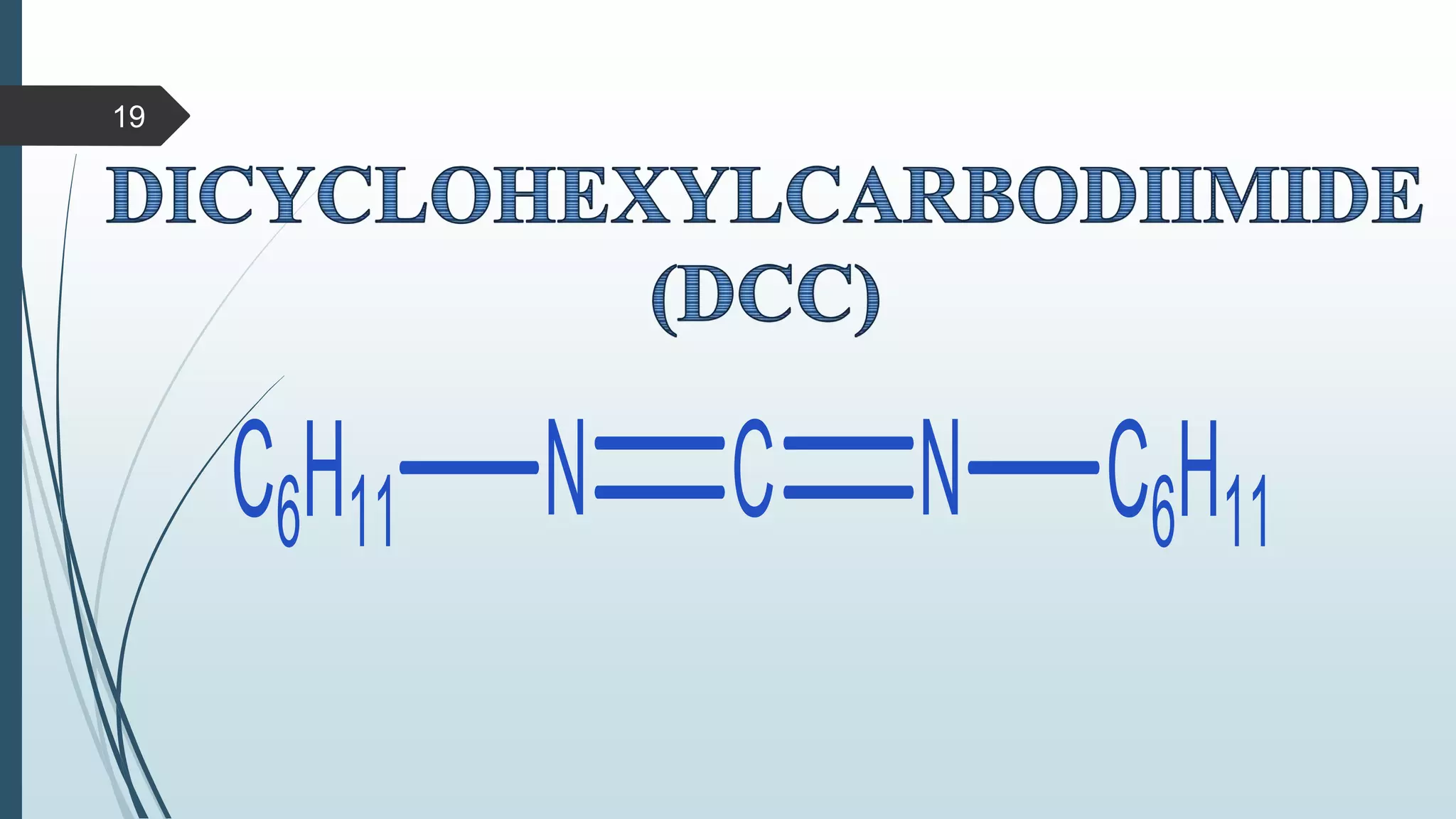
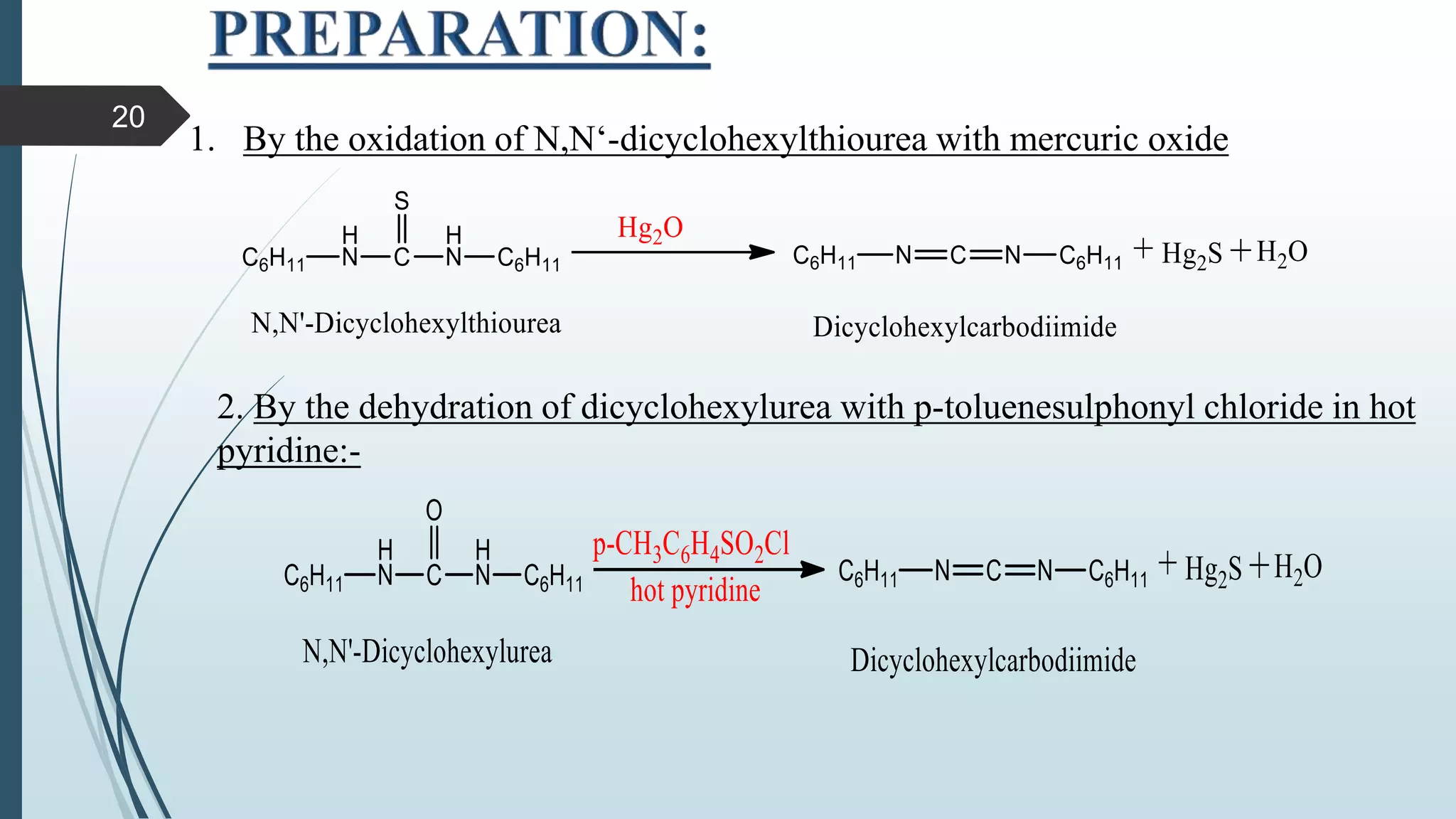
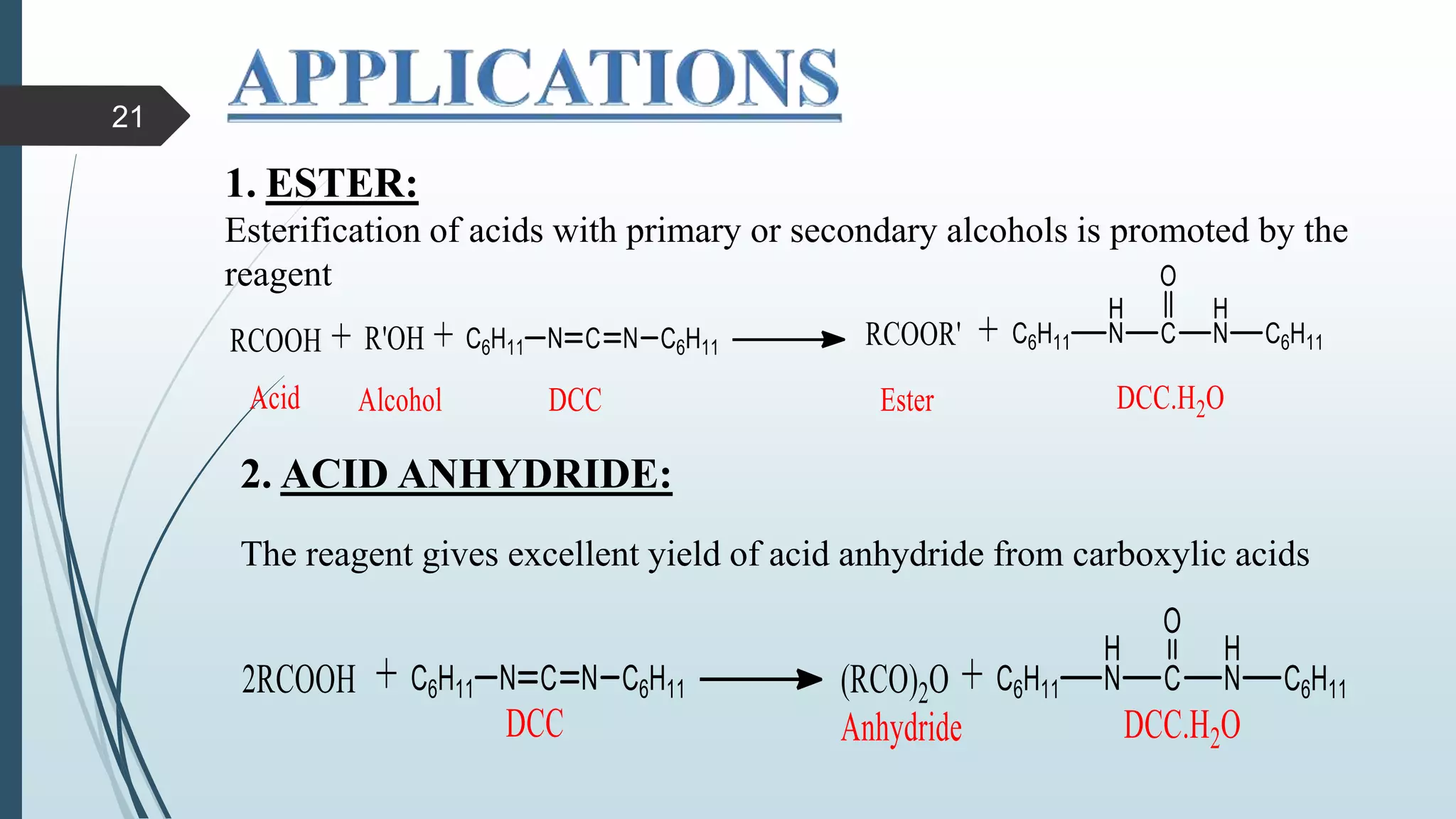
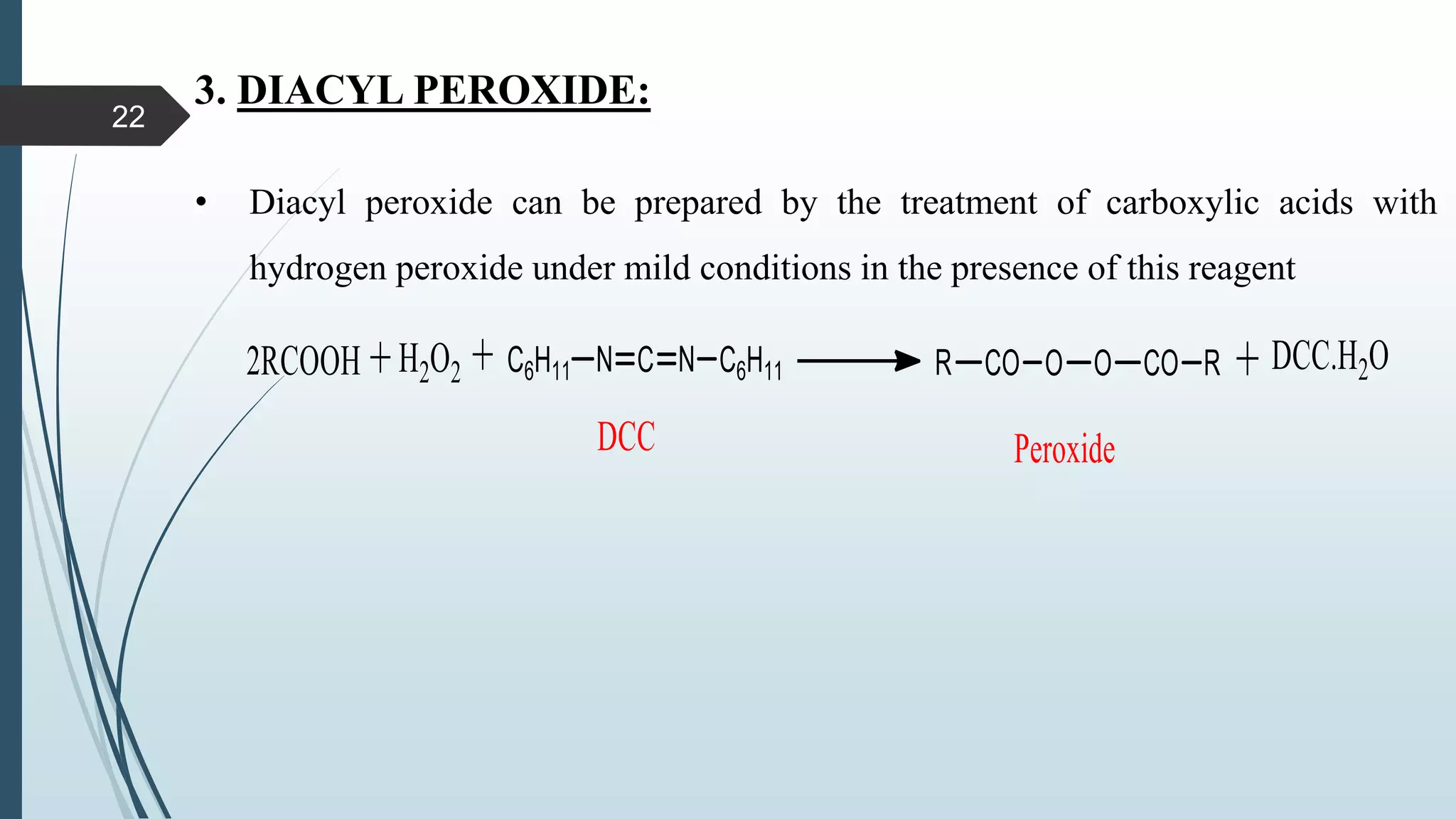
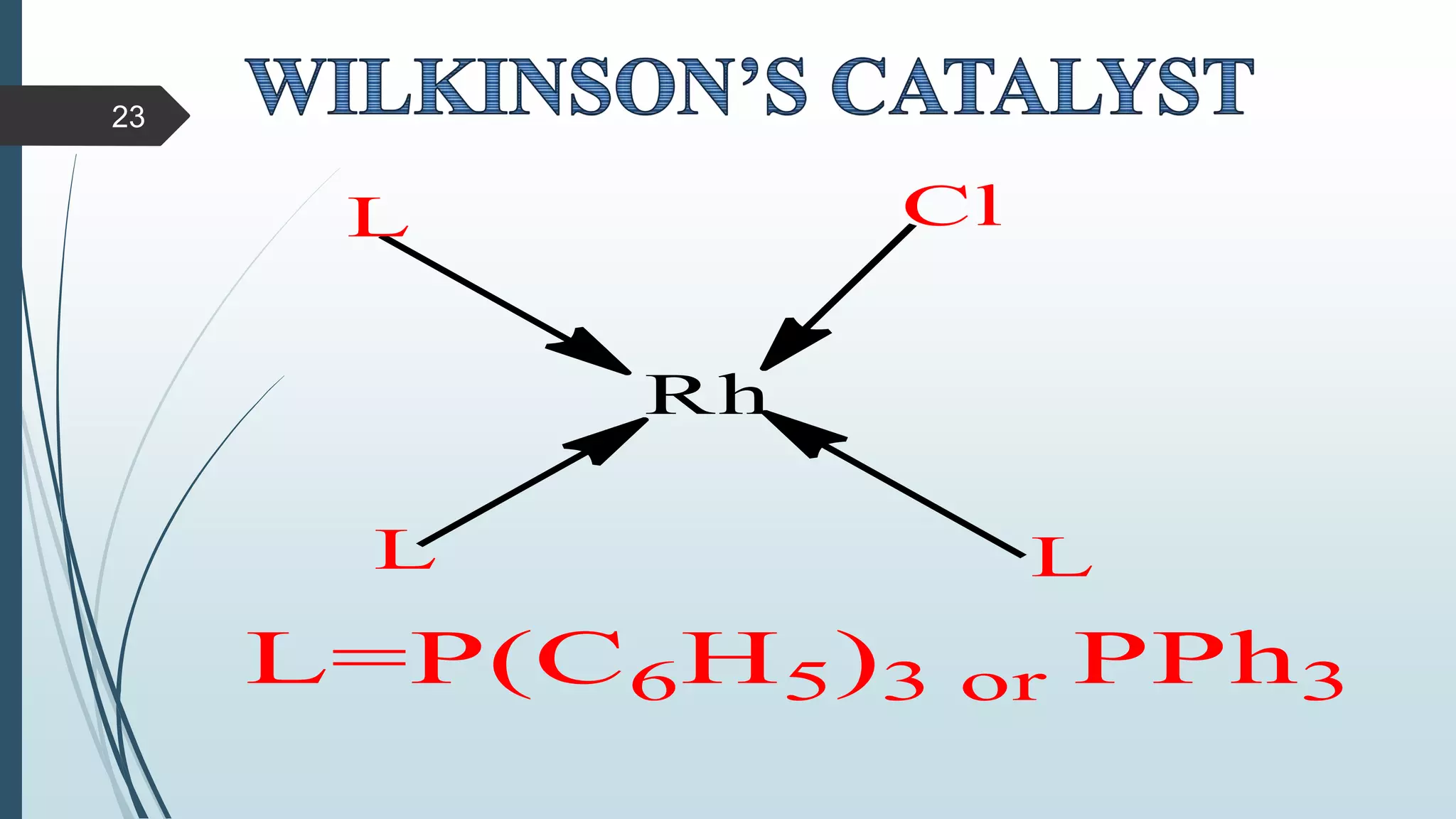
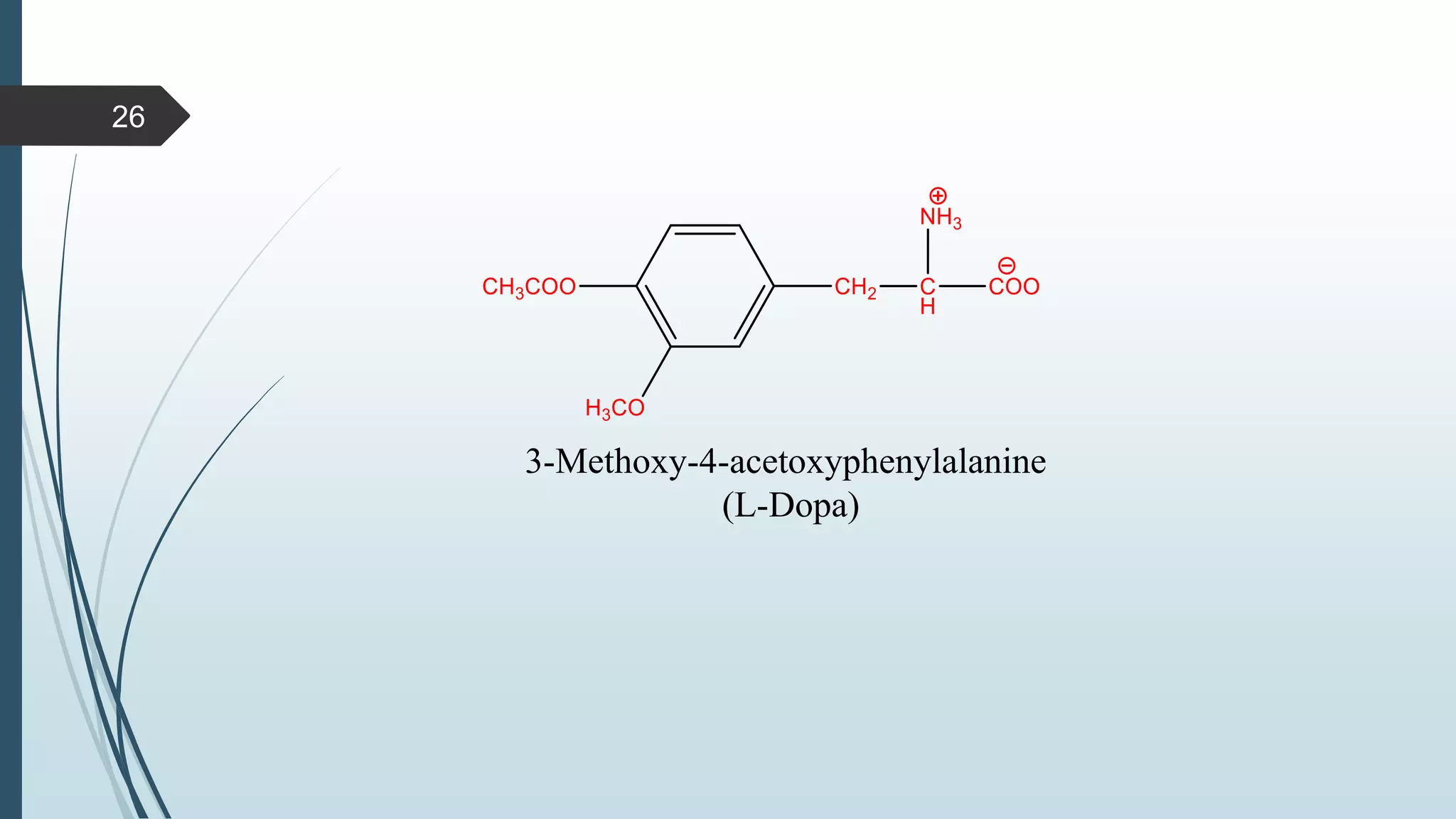
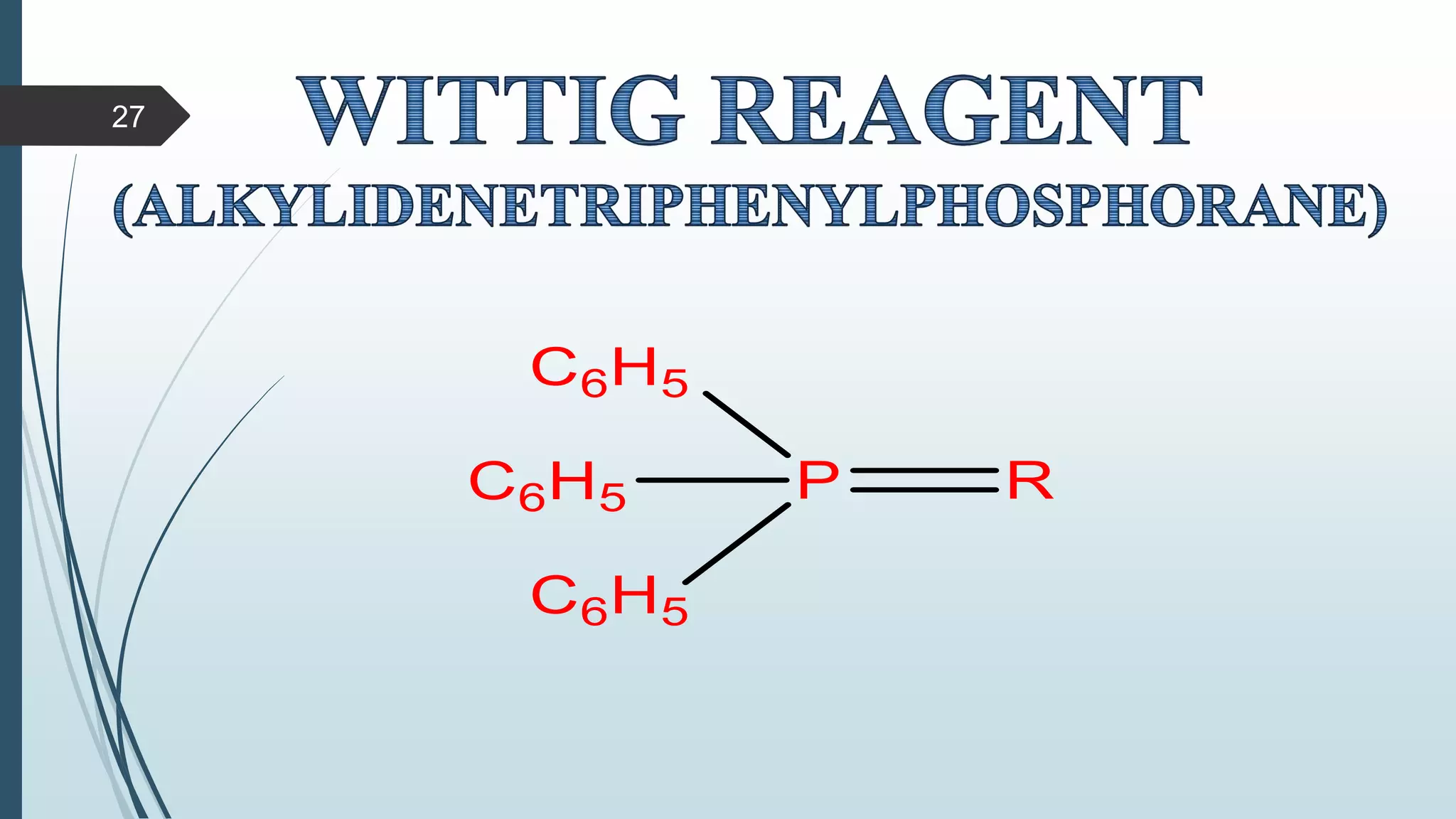
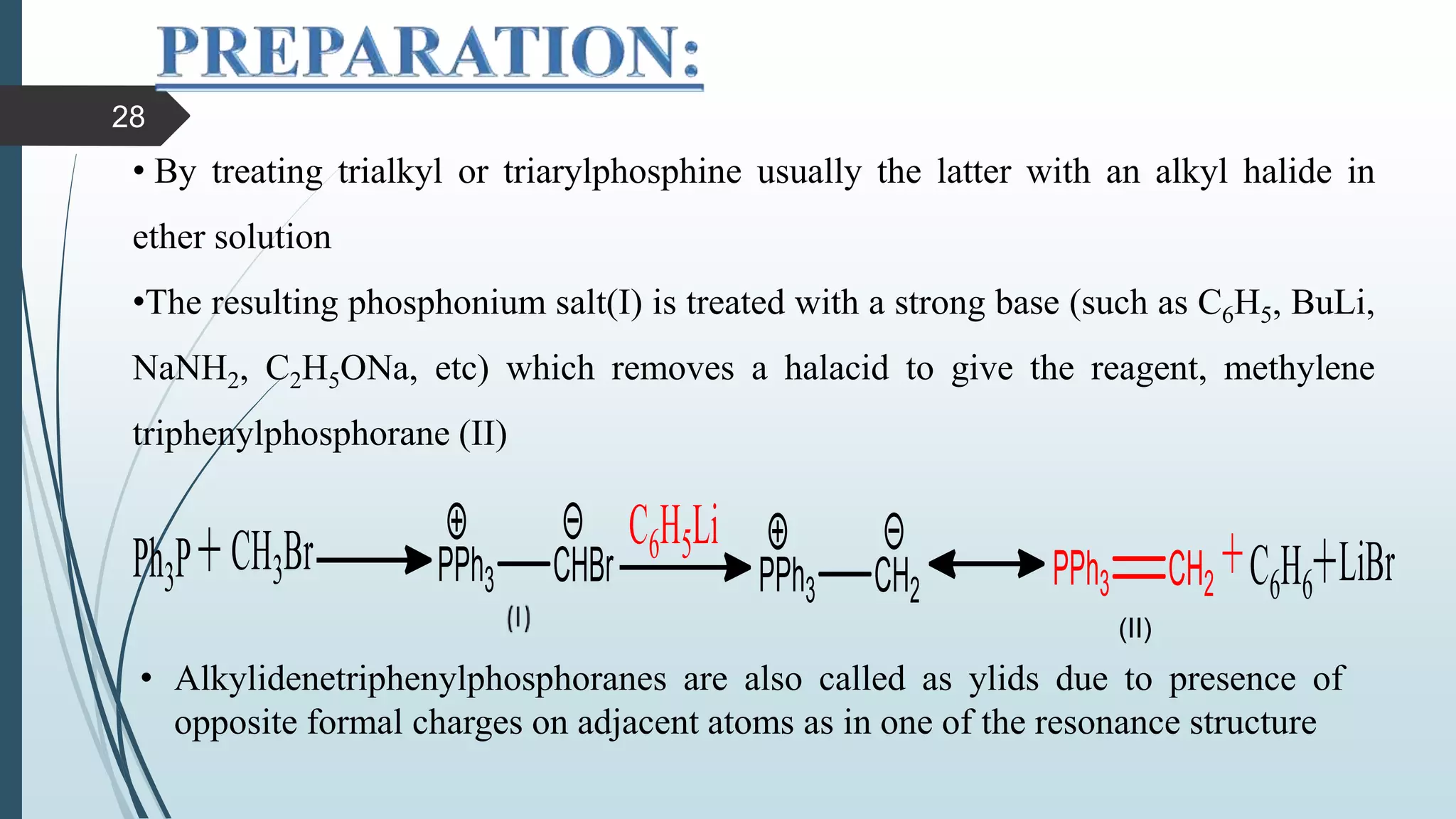
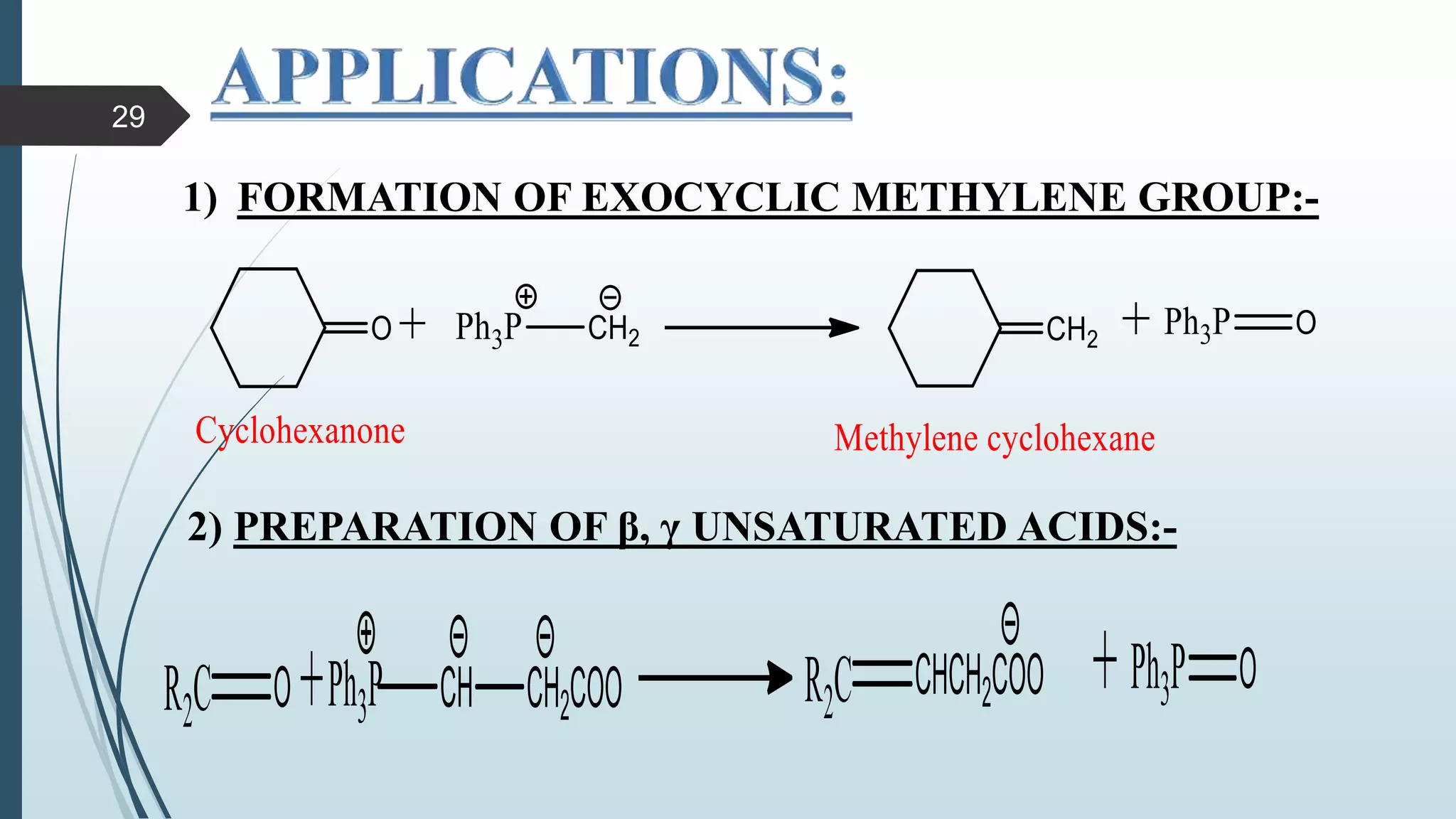
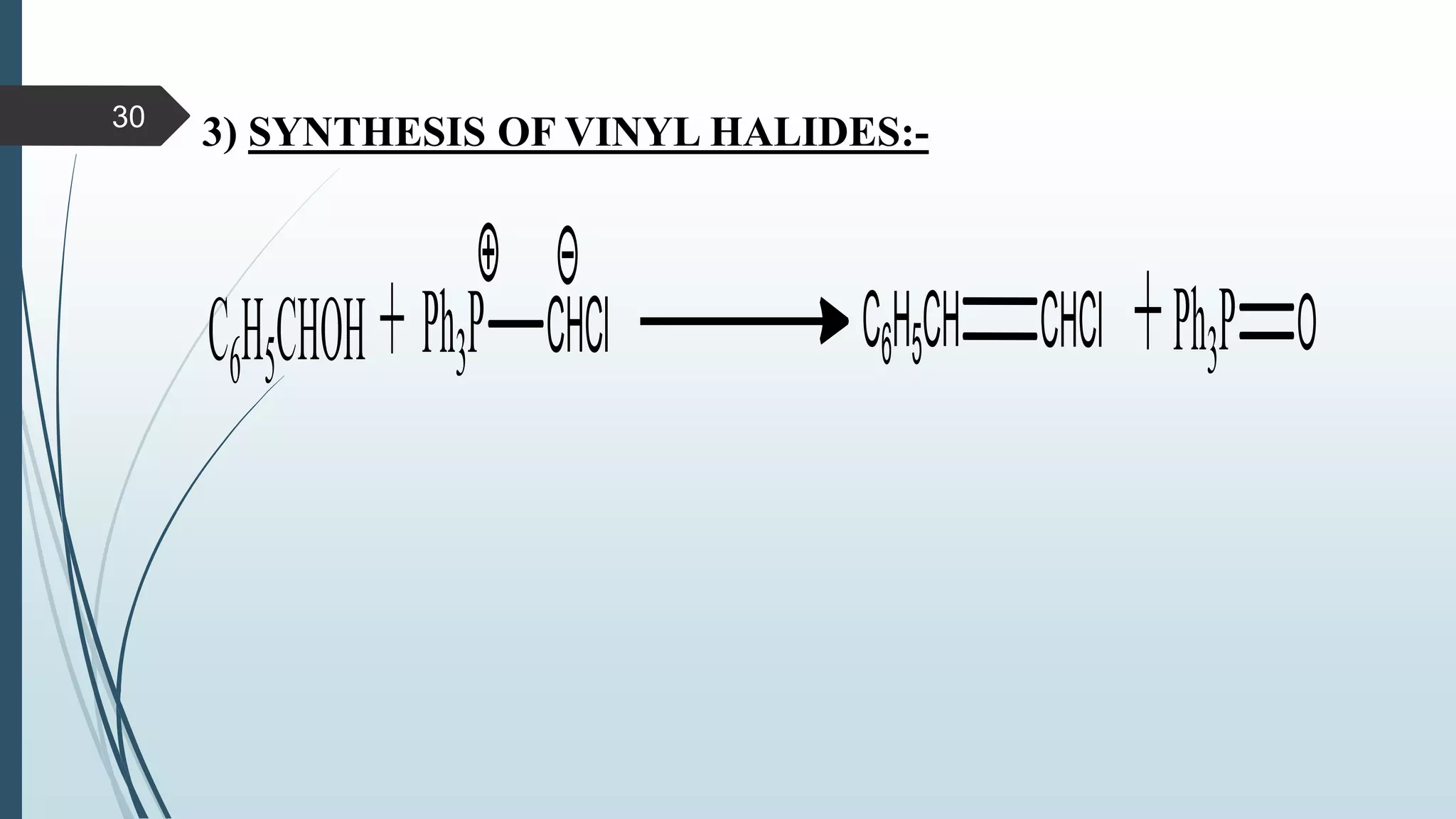
This document discusses several synthetic reagents and their applications. It introduces aluminum isopropoxide, N-bromosuccinamide, diazomethane, dicyclohexylcarbodiimide, Wilkinson reagent, and Wittig reagent. For each reagent, it provides information on preparation, reaction mechanisms, and common uses. The document aims to describe important reagents used in organic synthesis and their roles in producing natural products, pharmaceuticals, and industrial chemicals.