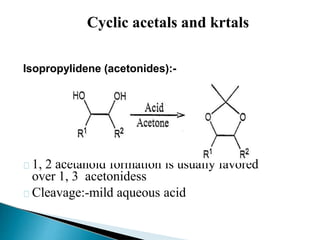
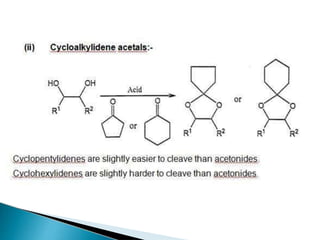
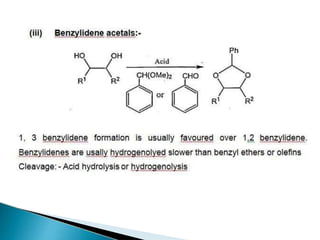
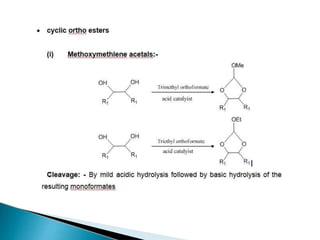
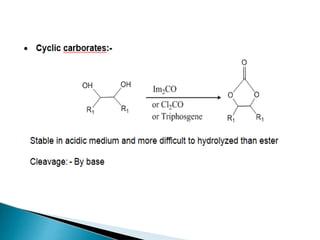
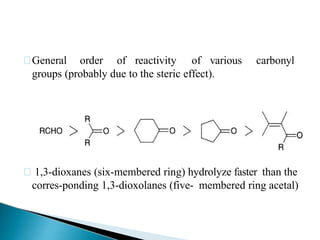
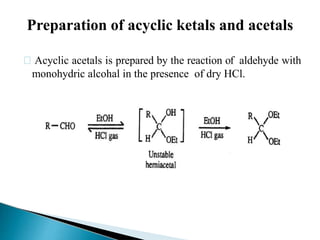
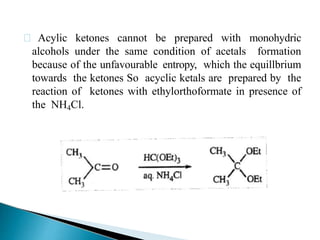
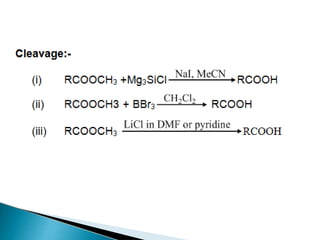
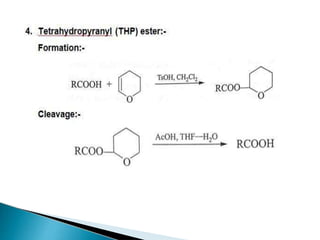
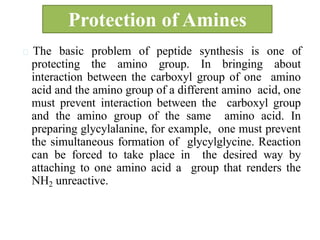
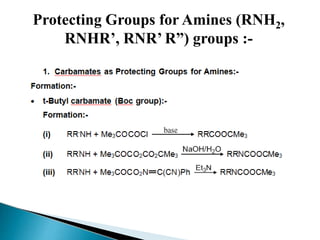
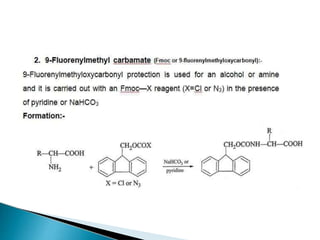
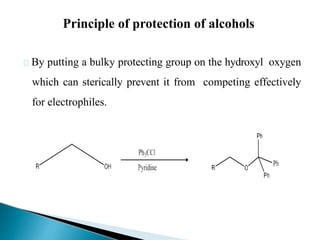
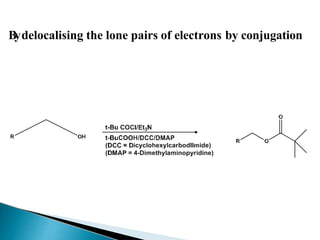
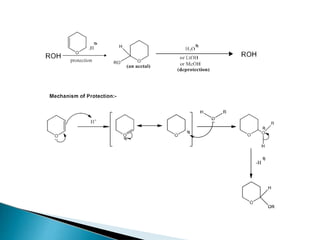
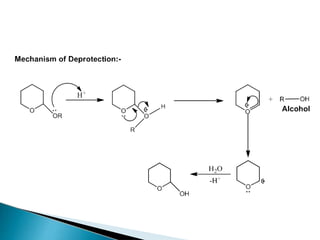
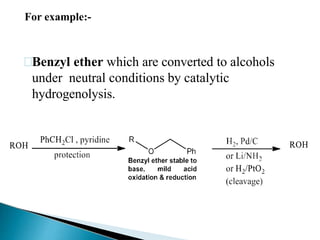
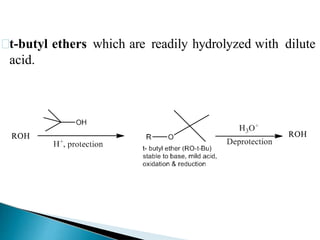
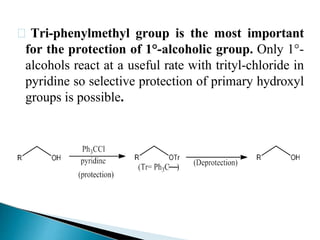
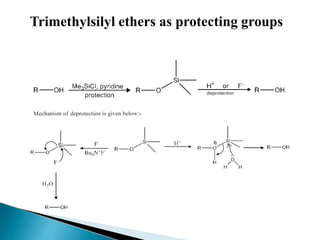
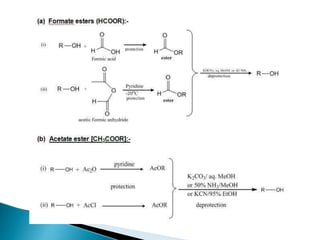
The document discusses the concept of protecting groups in organic chemistry, specifically their role in enabling selective reactions of less reactive functional groups. It details various types of protecting groups for alcohols, carbonyls, carboxylic acids, and amines, along with methods for their introduction and removal. Key considerations for choosing protecting groups include their availability, ease of use, stability, and efficiency in synthetic routes.










![Trichloroacetate esters
[Cl3CCOOR]:
Pivoloate ester
[Me3CCOOR]:-](https://image.slidesharecdn.com/rajnishkumarprotectinggroups-200321155107/85/Protecting-Groups-in-Organic-Chemistry-21-320.jpg)