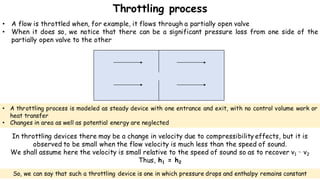
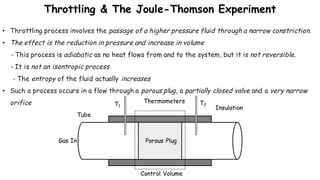
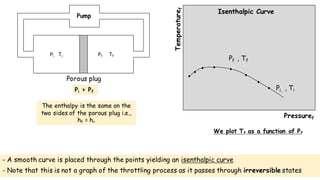
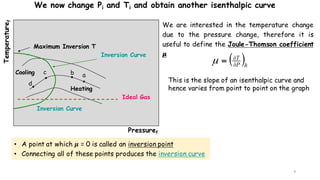
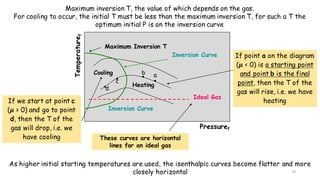
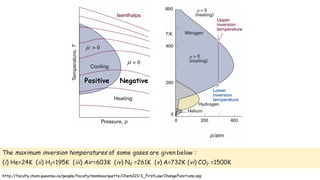
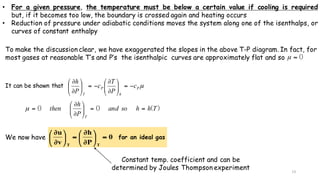
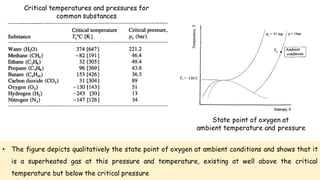
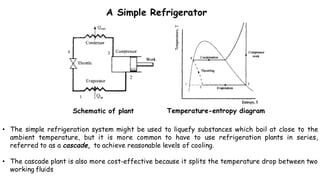
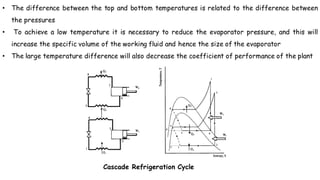
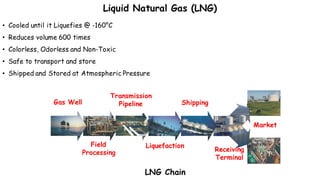
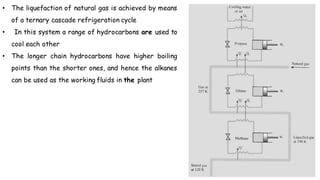
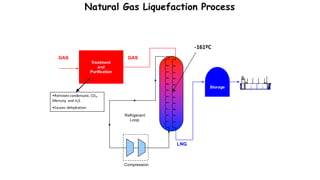
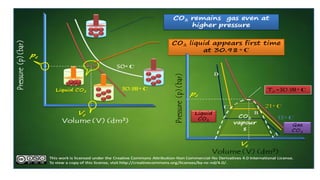
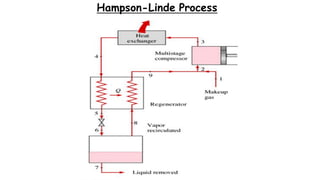
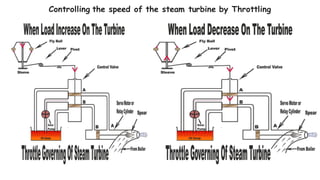
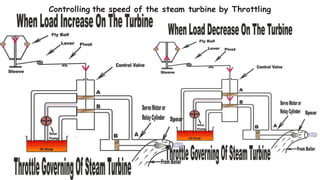
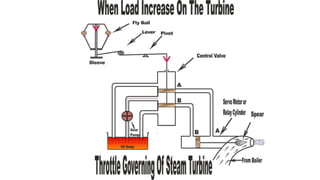
Throttling refers to the process where a fluid flows through a partially open valve, causing a significant pressure drop but constant enthalpy. During throttling, velocity may change slightly due to compressibility but enthalpy remains constant. Throttling is commonly used to control steam turbine speed, determine steam dryness, and in refrigeration plants and gas liquefaction. The Joule-Thomson experiment demonstrated that during throttling, enthalpy remains constant through a porous plug, validating the throttling process model.