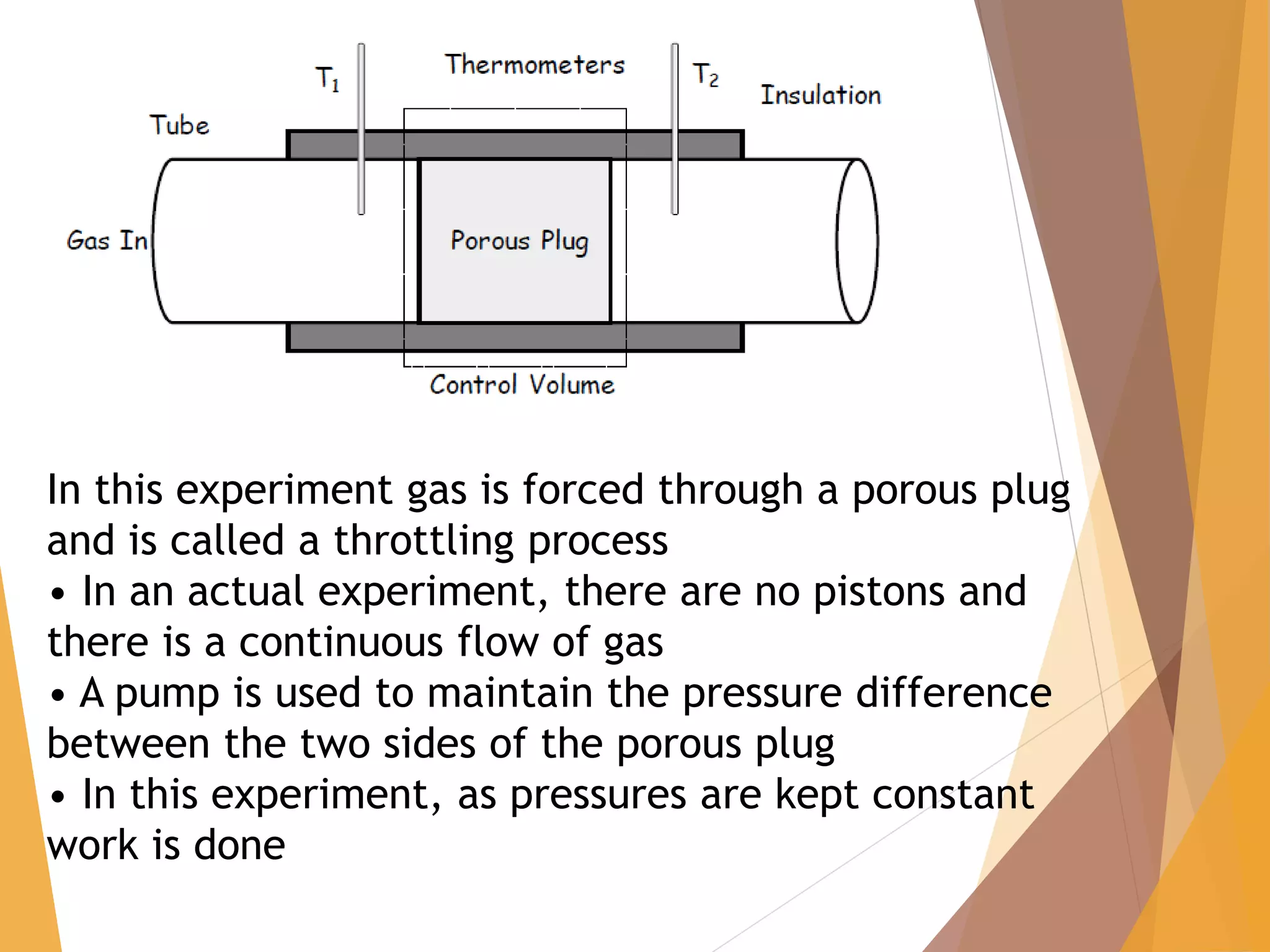
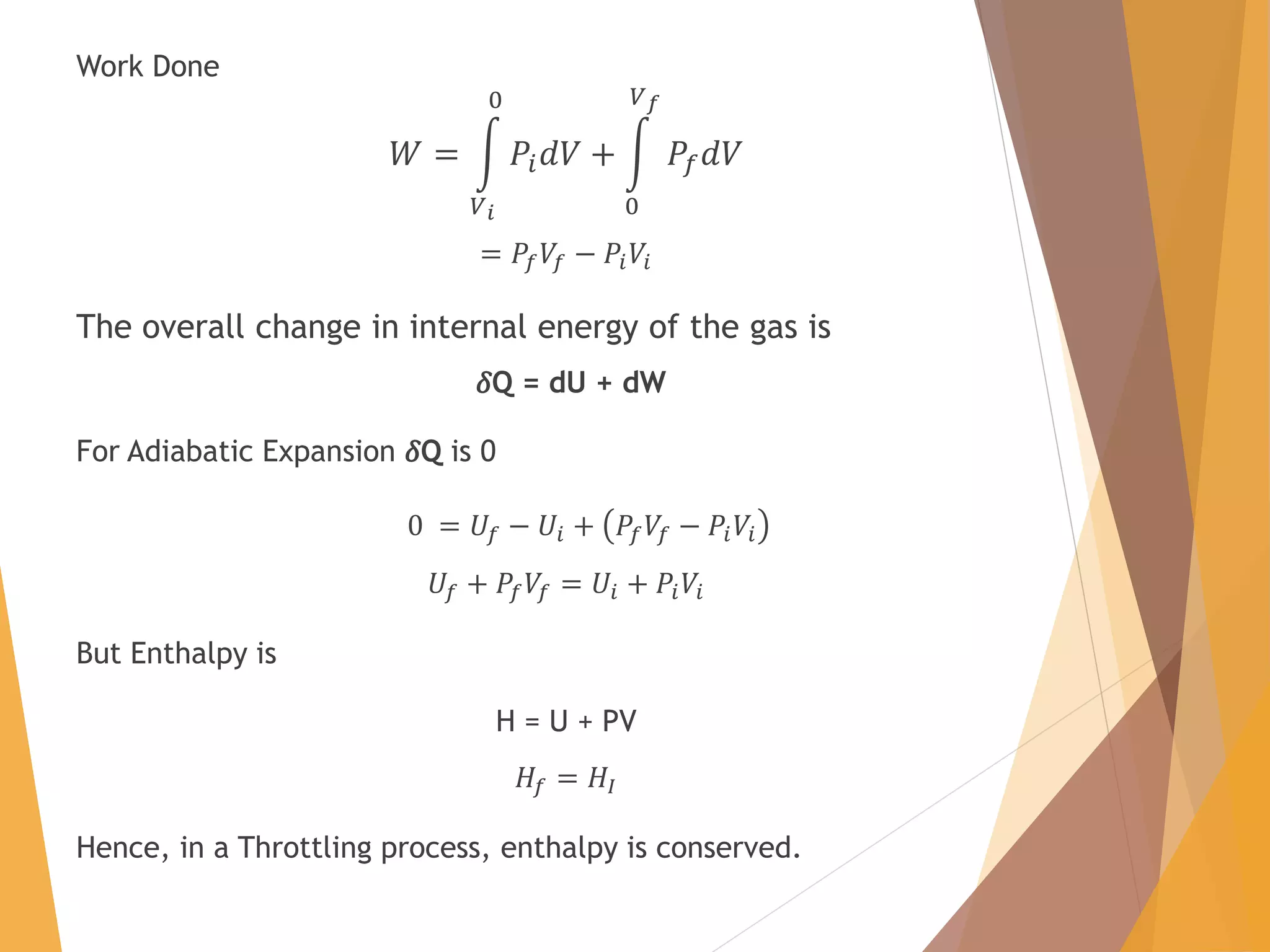
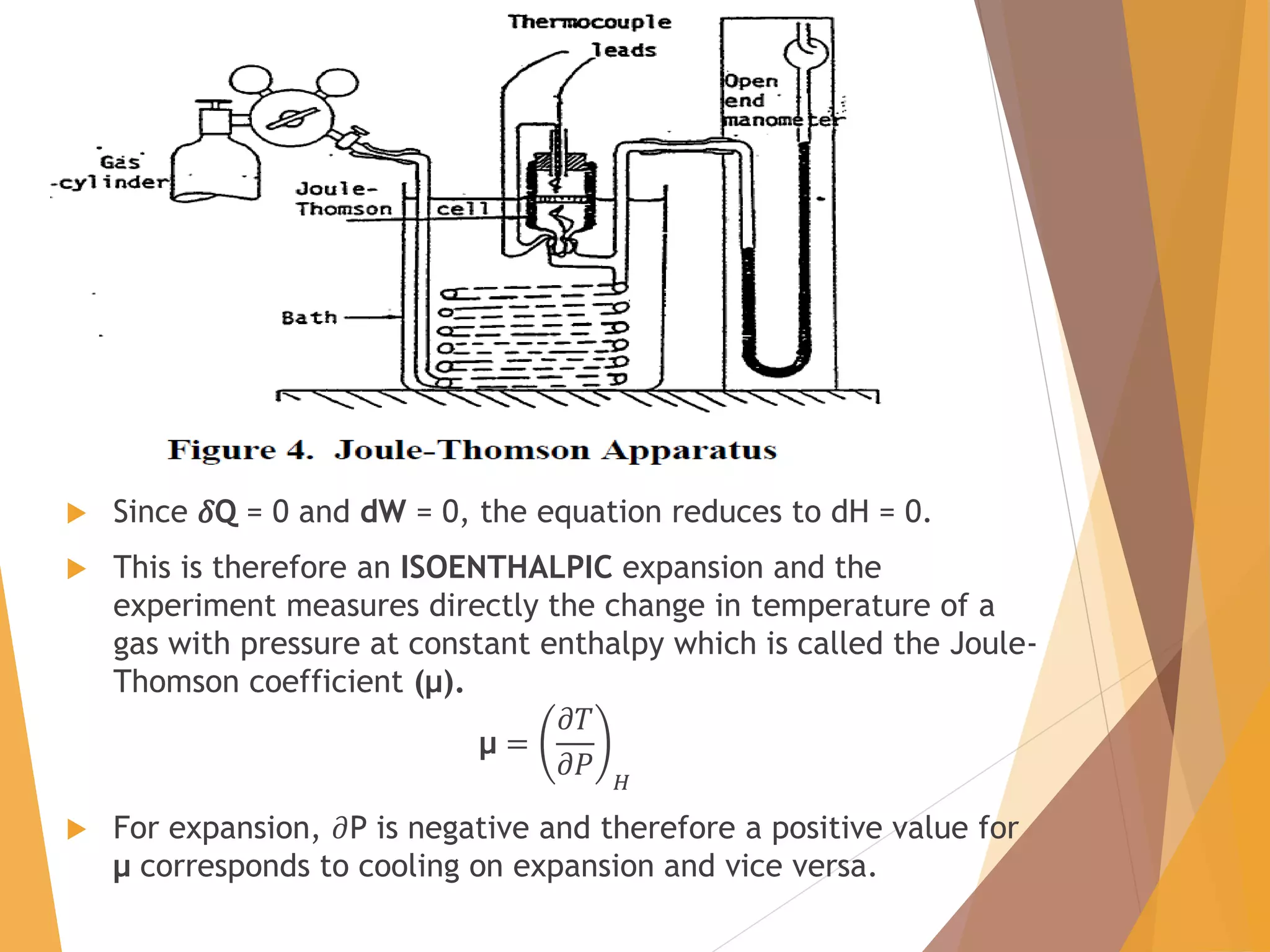
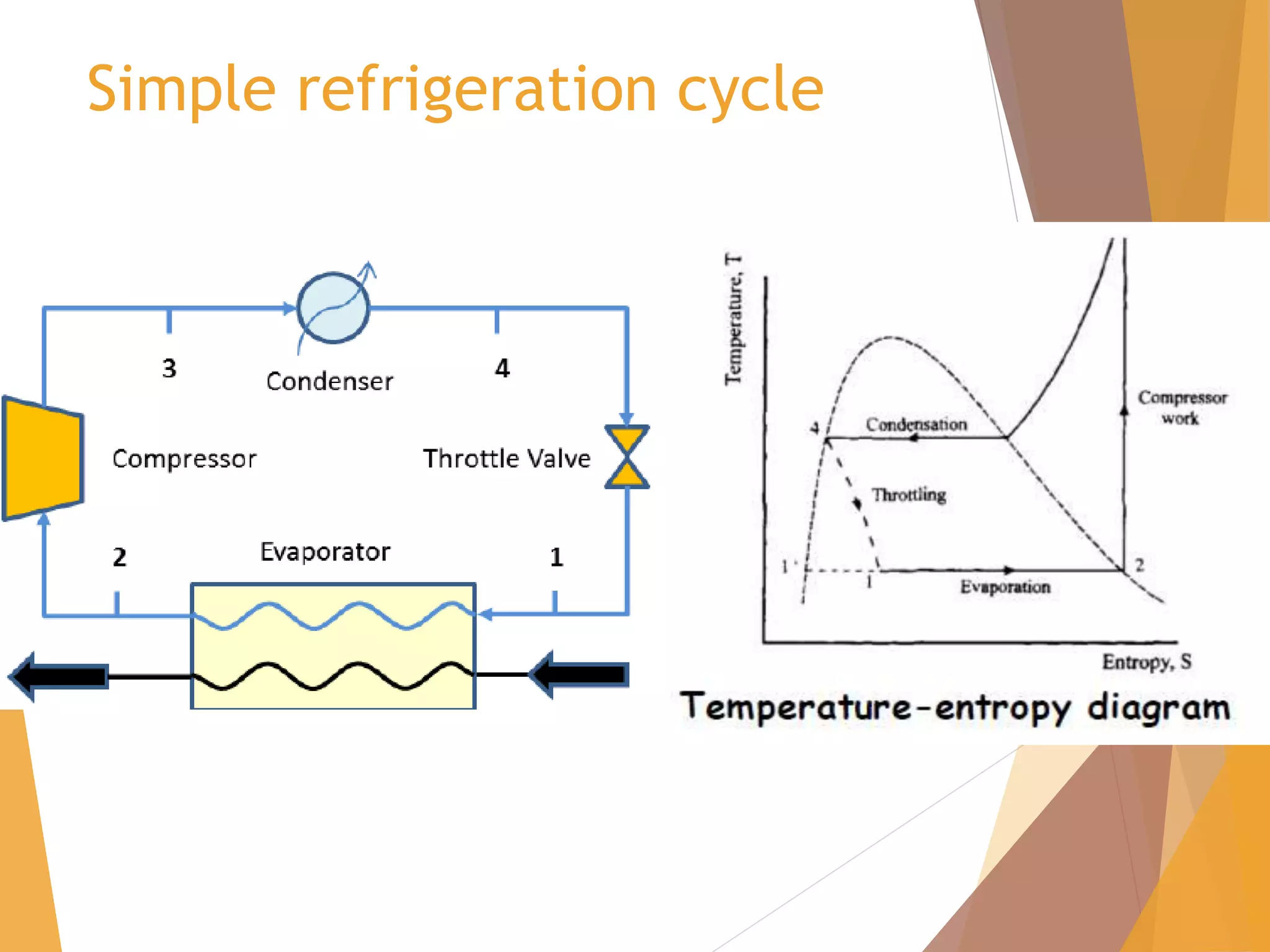
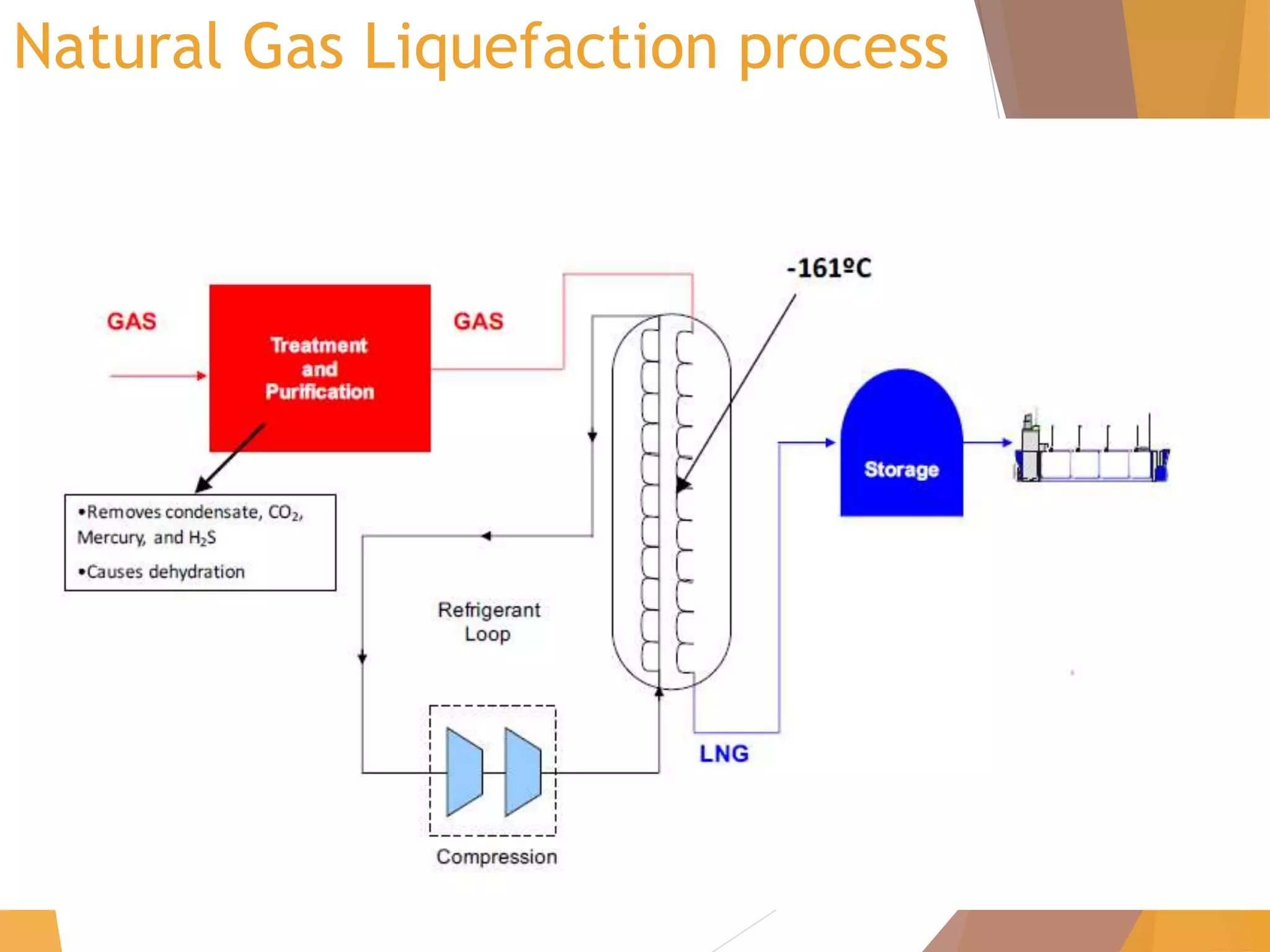
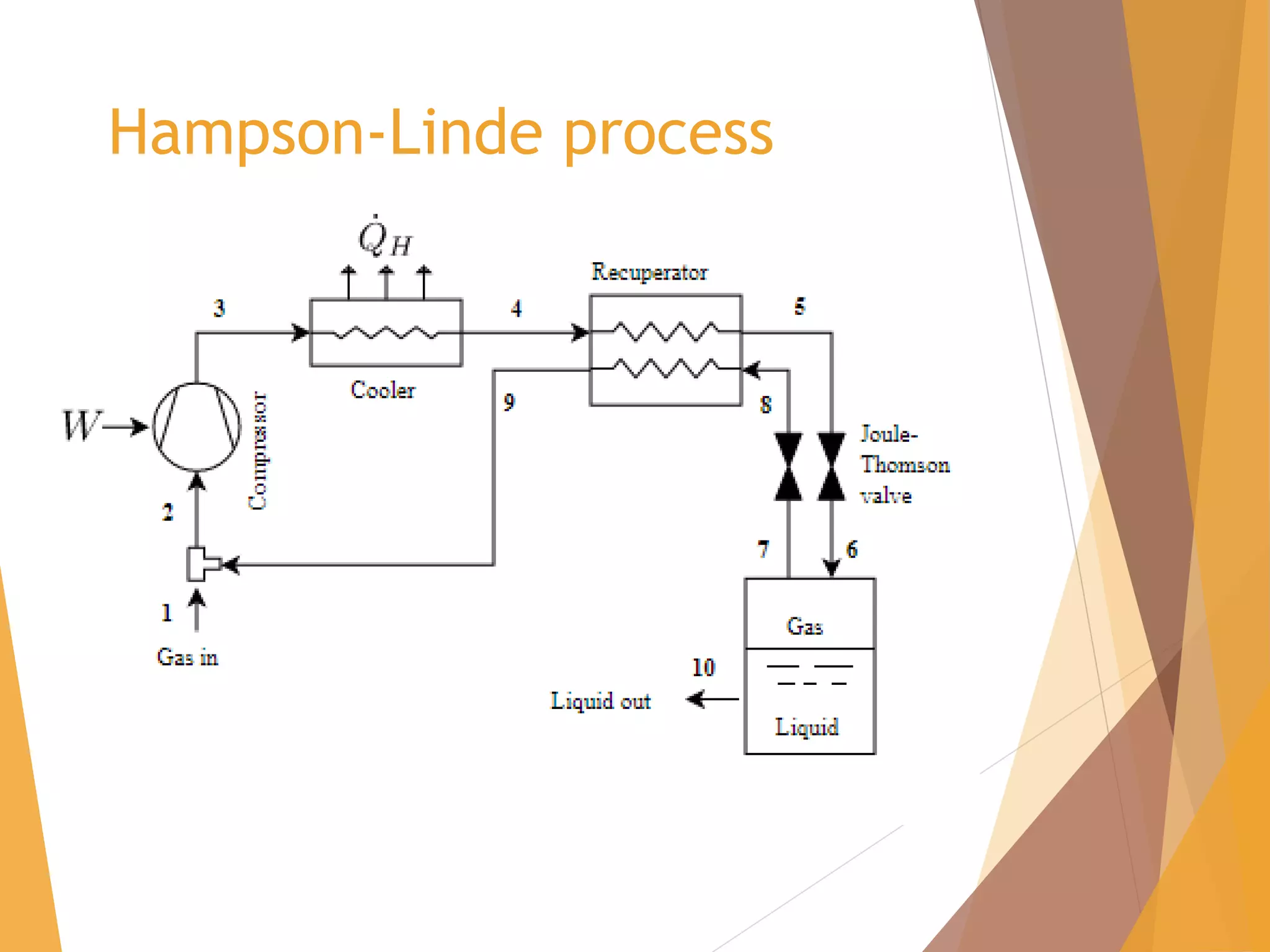
The document discusses throttling processes and the Joule-Thomson effect. It defines throttling as the passage of a fluid through a narrow constriction, causing a reduction in pressure and increase in volume. The Joule-Thomson experiment involves forcing a gas through a porous plug in a throttling process while maintaining constant pressures and measuring the temperature change. The experiment shows that for some gases, temperature decreases with decreasing pressure (positive Joule-Thomson coefficient), while for others it increases, depending on conditions. Applications of throttling discussed include determining steam dryness, controlling turbine speed, refrigeration, gas liquefaction, and cryogenic processes.