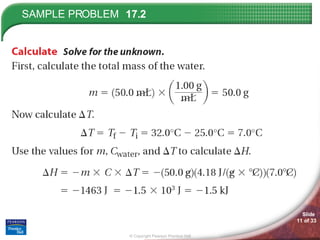
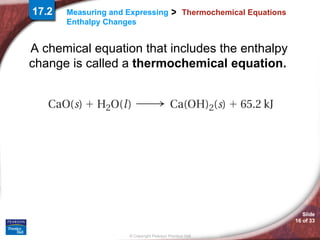
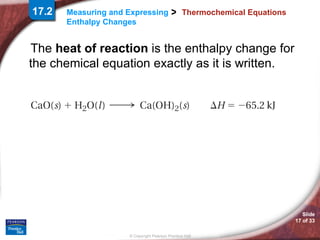
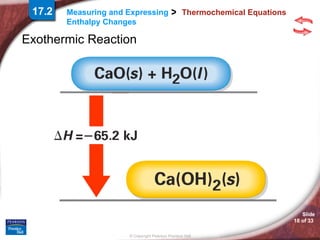
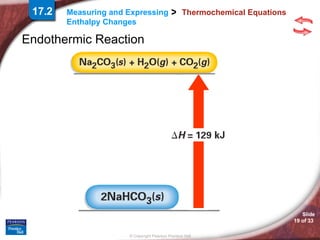
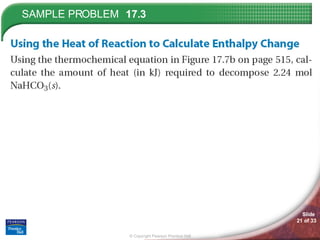
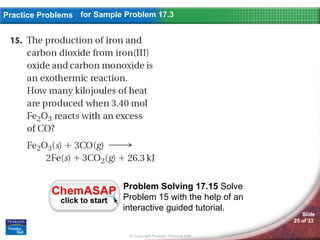
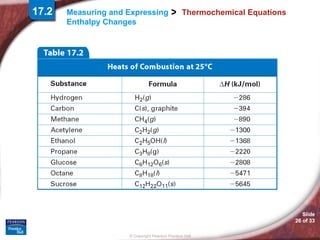
The document discusses calorimetry and measuring enthalpy changes. It defines calorimetry as the precise measurement of heat flow for chemical and physical processes using a calorimeter. A calorimeter is an insulated device that measures heat absorption or release. Calorimetry can be done at constant pressure or constant volume. Thermochemical equations include the enthalpy change of a reaction and express the heat of reaction.