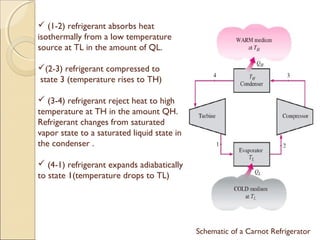
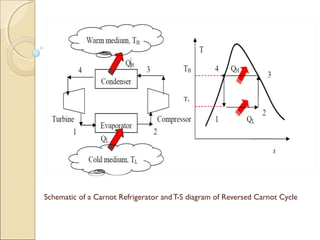
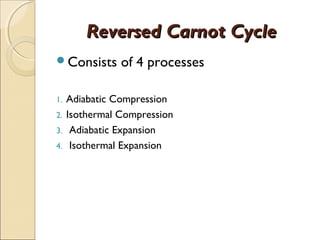
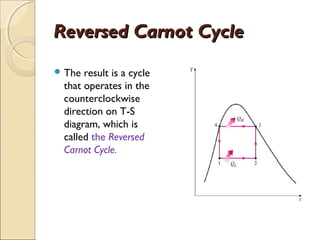
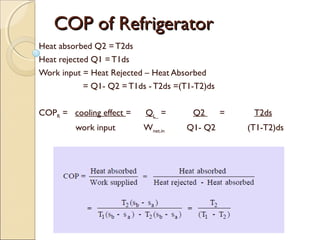
This document discusses the reversed Carnot cycle, which is used in Carnot refrigerators and heat pumps. It consists of four processes: 1) adiabatic compression, 2) isothermal compression, 3) adiabatic expansion, and 4) isothermal expansion. This cycle operates in the counterclockwise direction on a temperature-entropy diagram. It is the most efficient refrigeration cycle possible between two temperature levels, as it achieves the highest theoretical coefficient of performance. However, it cannot be practically implemented due to the different speeds required for the adiabatic and isothermal processes.