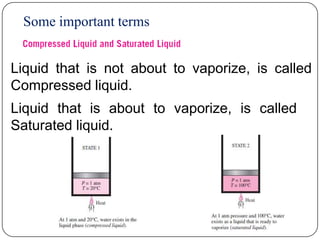
This document defines key terms related to steam formation and describes processes involved. It discusses:
- Terms like saturated liquid, saturated vapor, superheated vapor etc.
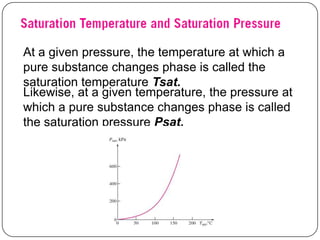
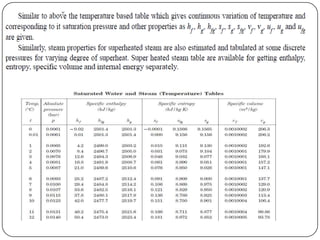
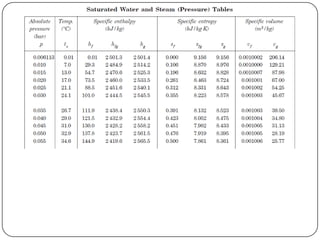
- Temperature and pressure where a substance changes phase (saturation temperature and pressure)
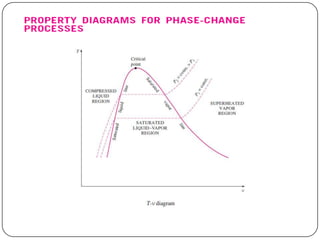
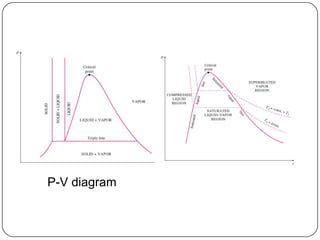
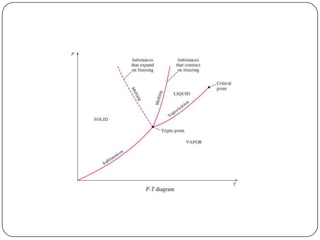
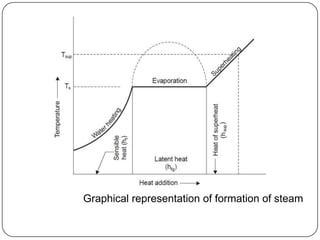
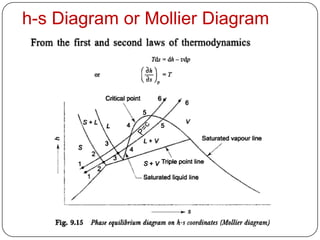
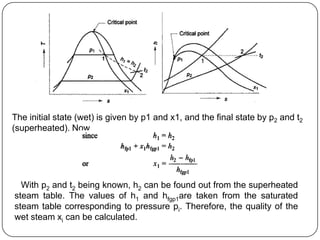
- Graphs like P-V and h-s diagrams that represent steam formation processes
- Quantities of heat absorbed during heating, vaporization (latent heat) and superheating of steam
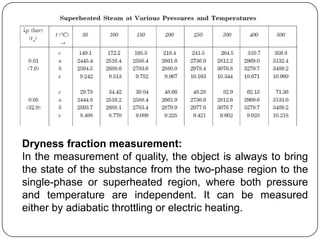
- Measurement of dryness fraction (quality) of wet steam using throttling or electric calorimeters.