1. The document discusses thermodynamics concepts including throttling process, heat engines, refrigerators, heat pumps, and the first and second laws of thermodynamics.
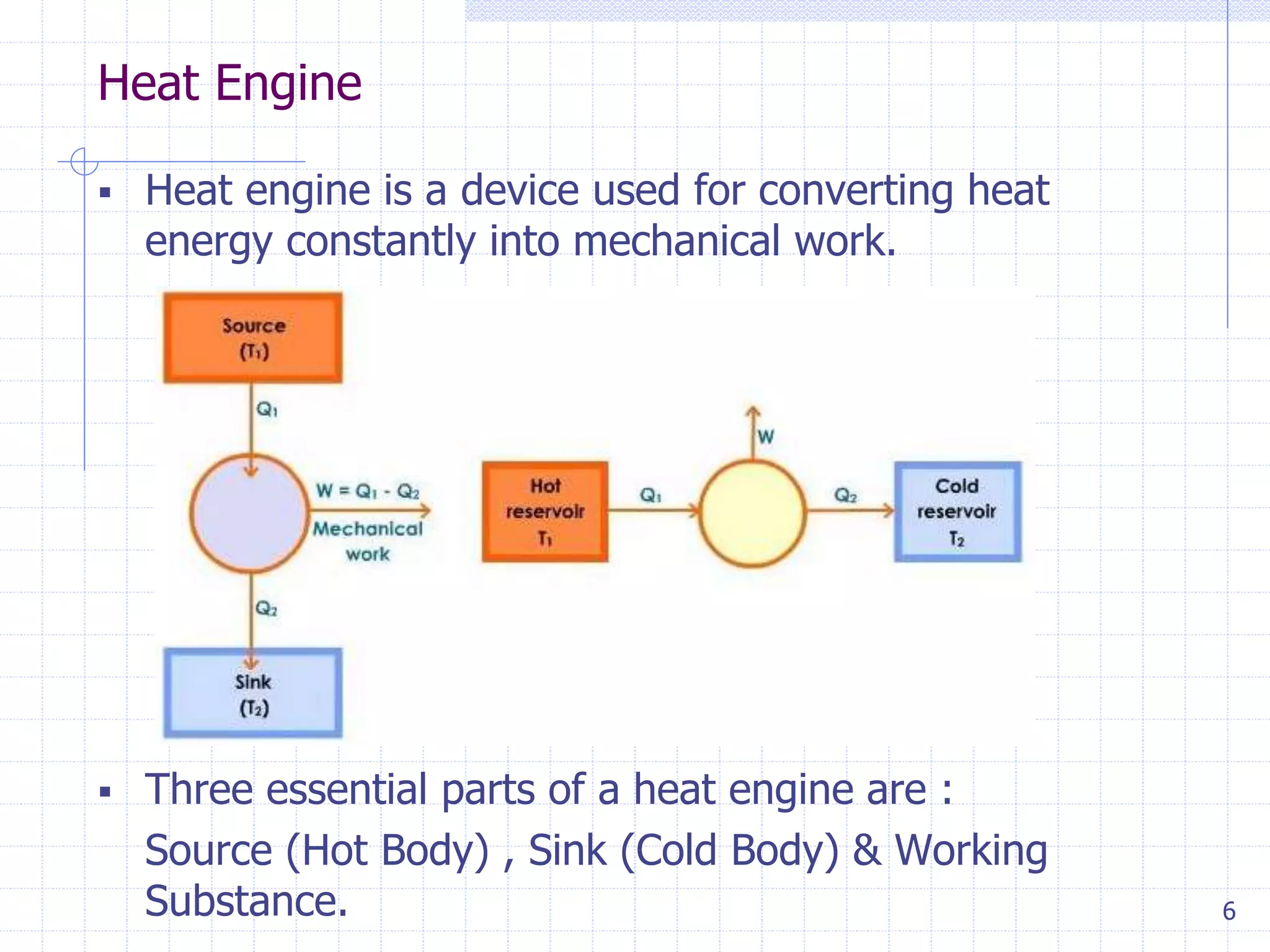
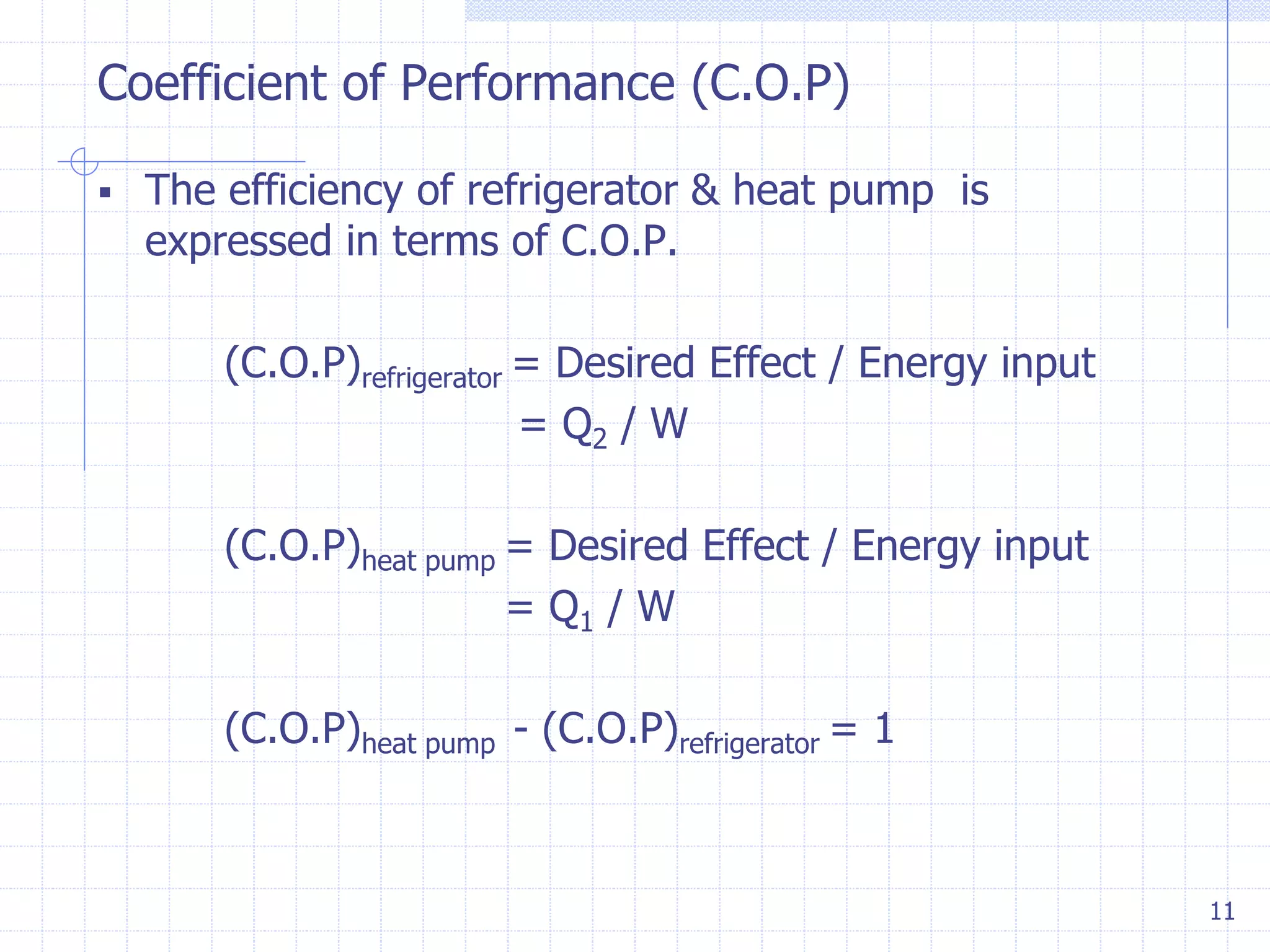
2. It defines key terms like throttling process, free expansion, heat source, heat sink, heat reservoir, thermal efficiency, and coefficient of performance.
3. Several statements of the second law are provided including Clausius statement and Kelvin-Planck statement.