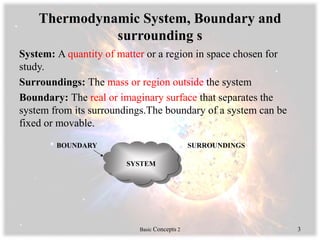
This document provides an overview of basic thermodynamics concepts including:
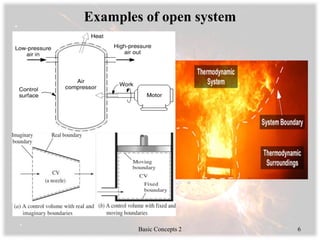
- Systems, boundaries, surroundings, and the types of thermodynamic systems such as closed, open, isolated, and rigid.
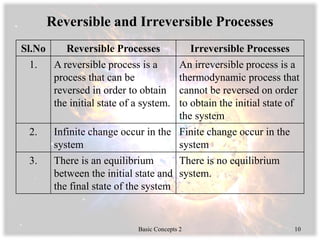
- Thermodynamic states, processes, paths, and cycles along with examples of different processes.
- The basic definitions of heat, work, internal energy, and enthalpy along with the sign conventions.
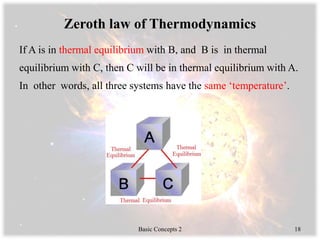
- The zeroth law of thermodynamics regarding thermal equilibrium and temperature.
- The first law of thermodynamics regarding the relationship between heat, work, and changes in internal energy for closed and open systems.








![Work (W)
1.Displacement Work ,Wd = Force(F) Distance(dx)
W = p.A.dx [Pressure, p= F/A]
W= ʃ p.dv
1 J = 1 N∙ m : 1 cal = 4.1868 J
1 Btu = 1.0551 kJ
Work done = Area under a p-V curve
W > 0 Work done by the system (+ve) or Expansion
W < 0 Work done on the system(-ve) or Compression
Basic Concepts 2 13](https://image.slidesharecdn.com/gteqst5qt6szjfjtxsa0-signature-cb893d1636aa2bab99081ac078797160df3623679bfcbba2251571ec4a529d96-poli-180630100932/85/Engineering-Thermodynamics-Basic-Concepts-2-13-320.jpg)