The document provides a comprehensive overview of animal tissue culture, focusing on primary cultures and cell lines. It details the steps involved in creating and maintaining primary cultures, including tissue isolation and disaggregation methods, as well as the characteristics of finite and continuous cell lines. Additionally, it discusses considerations for choosing and naming cell lines, along with maintenance requirements.








![Primary Explant Culture
• The primary explant technique was the original
method developed by Harrison [1907], Carrel
[1912].
• As originally performed, a fragment of tissue
was embedded in blood plasma or lymph,
mixed with heterologous serum and embryo
extract, and placed on a cover slip that was
inverted over a concavity slide.](https://image.slidesharecdn.com/primarycultureandcellline-200515065703/85/Primary-culture-and-cell-line-9-320.jpg)




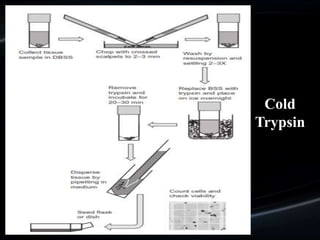

![spillage: collecting the cells
that spill out when the tissue
is carefully sliced and the slices
scraped [Lasfargues, 1973].
sieving: pressingthe dissected
tissue through a series of
sieves for which the mesh is
gradually reduced in size.
syringing: forcing
the tissue fragments through a
syringe (with or without a
wide-gauge needle) [Zaroff et
al., 1961]
pipetting
MECHANICAL
DISAGGREGATION](https://image.slidesharecdn.com/primarycultureandcellline-200515065703/85/Primary-culture-and-cell-line-16-320.jpg)







