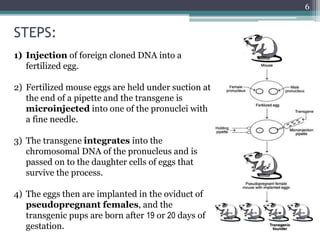
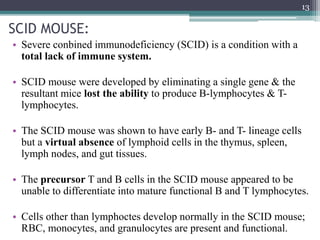
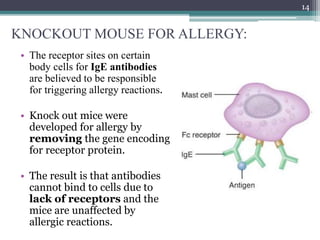
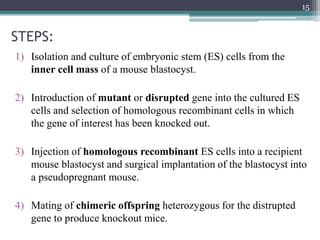
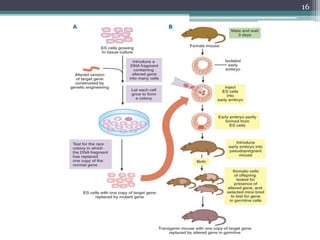
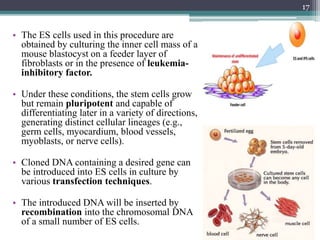
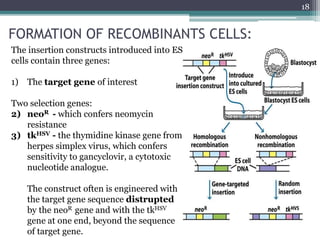
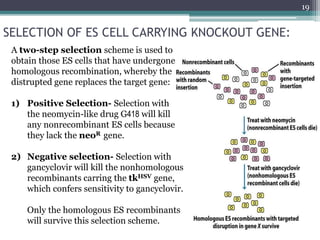
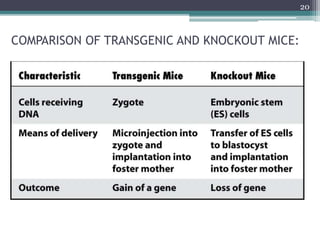
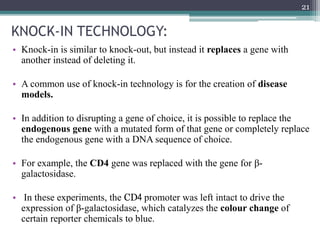
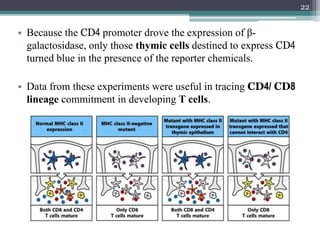
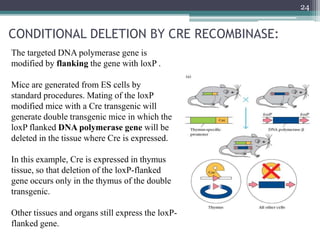
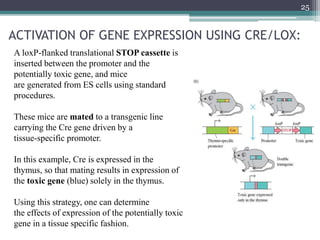
The document discusses transgenic and knockout mice, focusing on methods for producing transgenic mice, including retroviral vector, microinjection, and embryonic stem cell methods. Transgenic mice serve as valuable tools for studying gene function and modeling human diseases, while knockout mice provide insights into gene roles by disabling specific genes. The document also considers advanced techniques such as inducible knockout systems and the limitations of current knockout models.