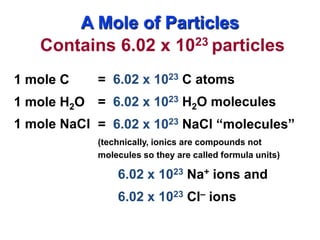
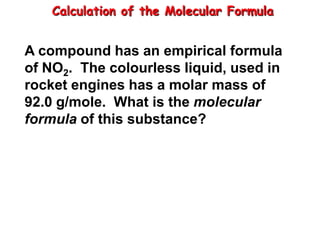
The document discusses moles, which are a unit used to measure very large quantities in chemistry. A mole is defined as 6.02 x 1023 particles, such as atoms, molecules, ions. This number is known as Avogadro's number. The document provides examples of how many everyday items would equal a mole to demonstrate just how large a mole is. It also discusses how moles can be used to convert between the number of particles, moles, and mass in grams.