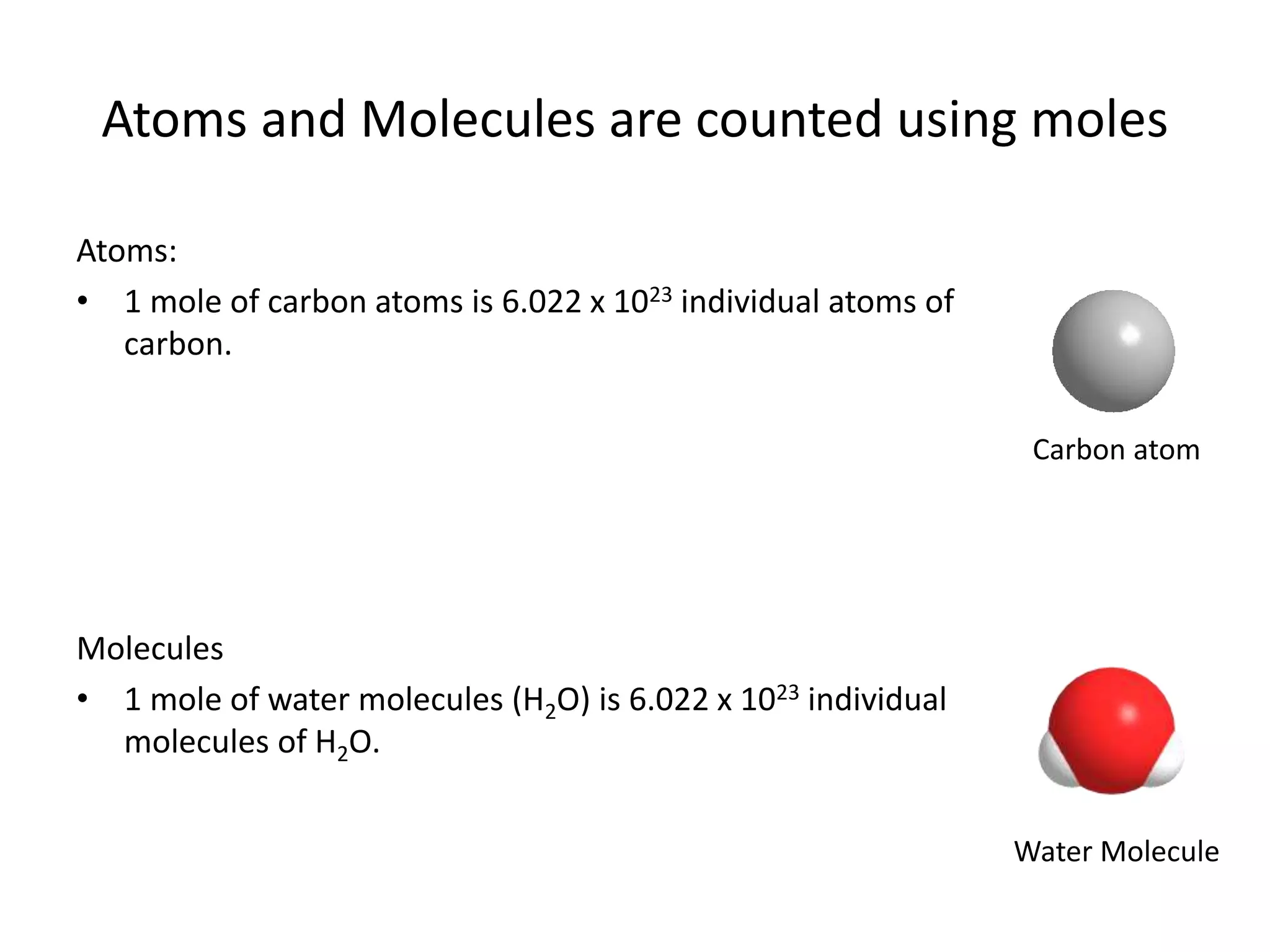
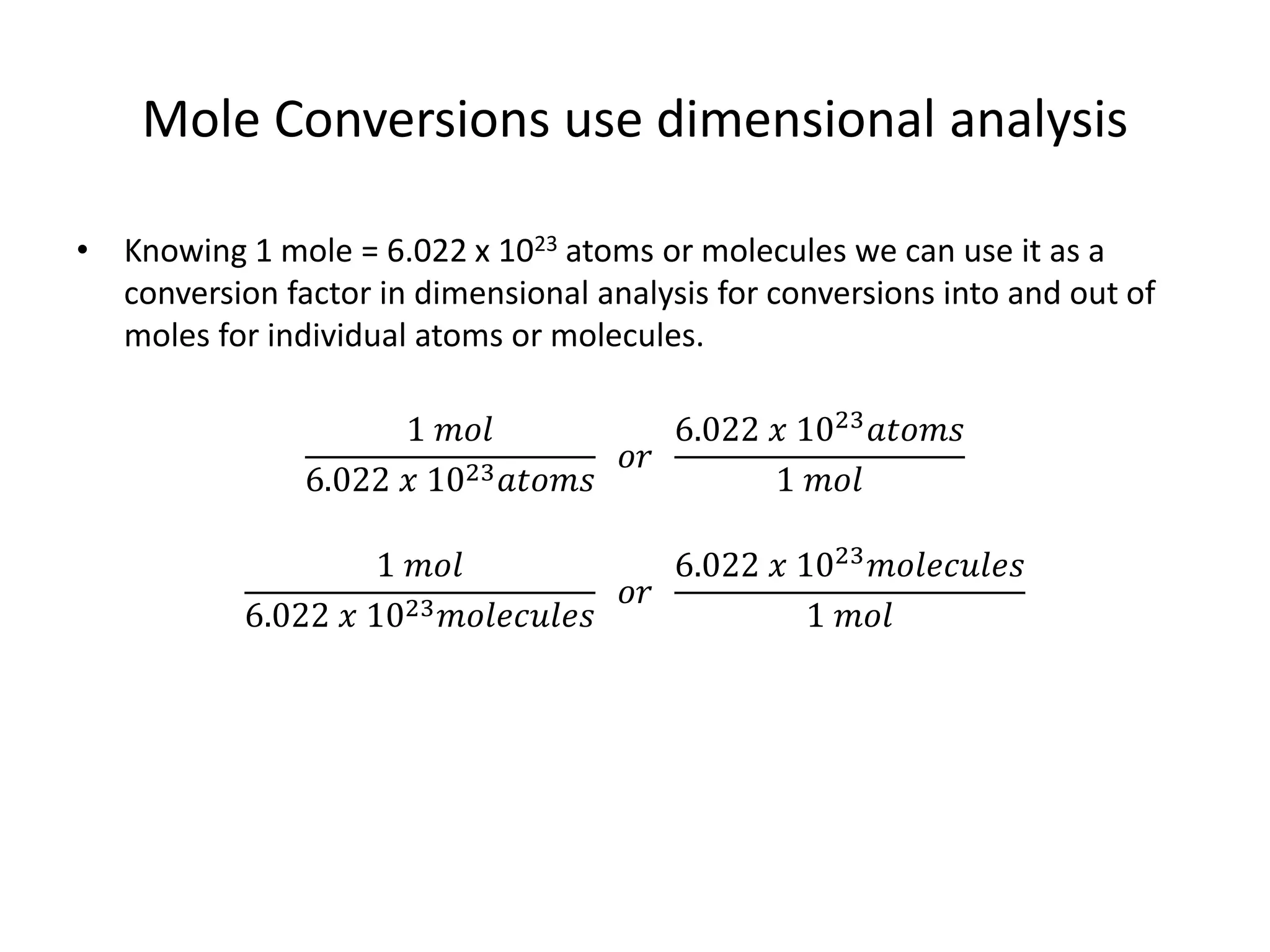
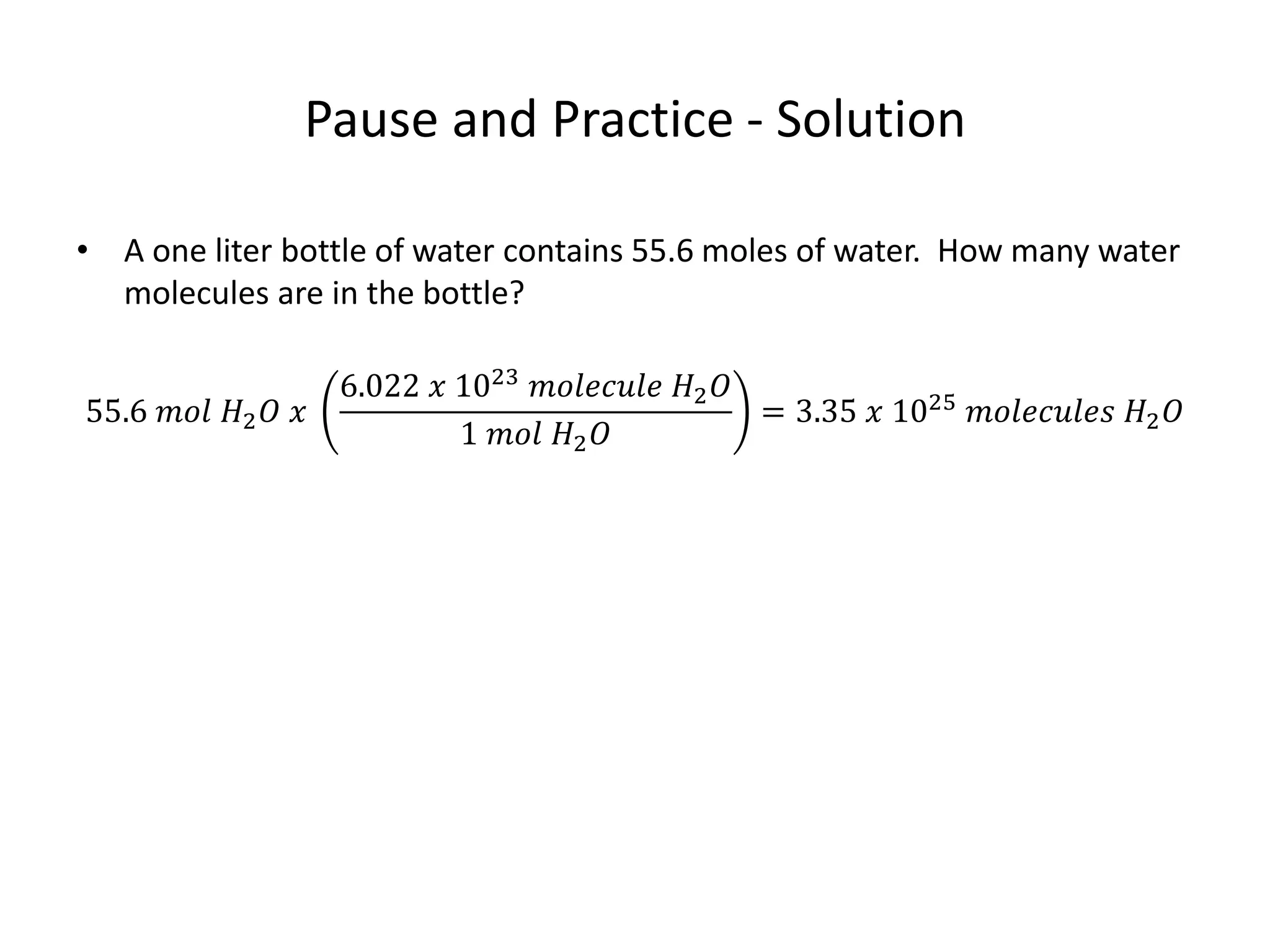
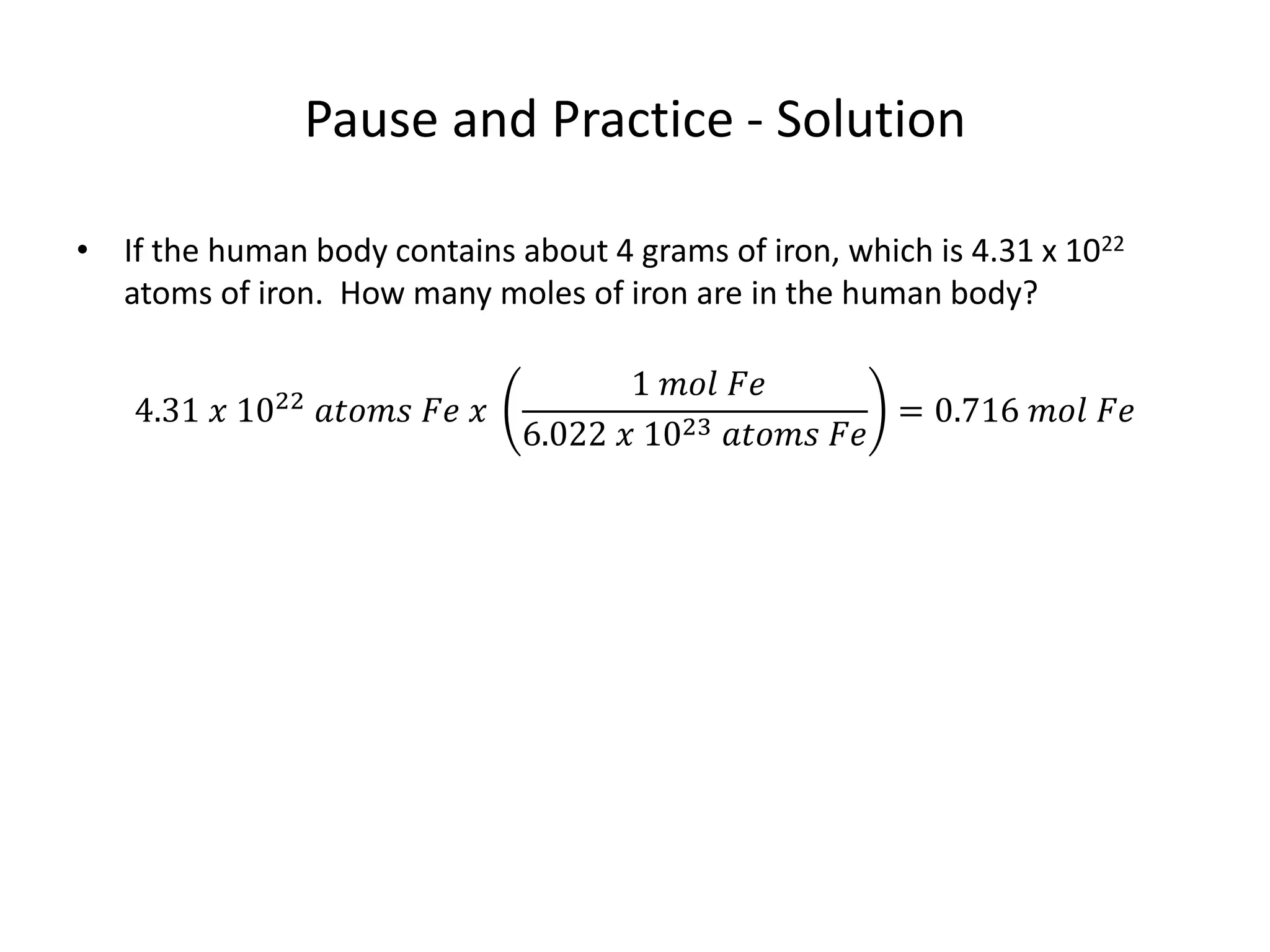
The document discusses the mole as a unit used to quantify atoms and molecules. It explains that atoms and molecules are too small to count individually, so they are grouped into moles containing 6.022 x 1023 particles. This allows for easier calculations involving large numbers of tiny particles. Examples show how moles can be used to determine the number of individual atoms or molecules present. The mole is analogous to counting items in dozens, and conversions between moles and individual particles use the mole-to-particle conversion factor.