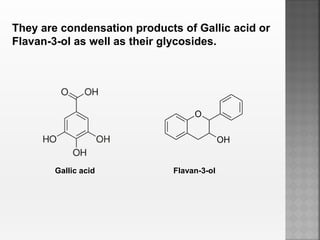
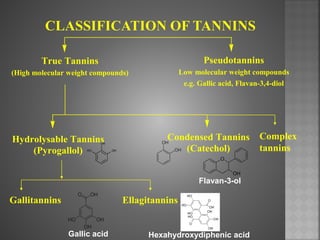
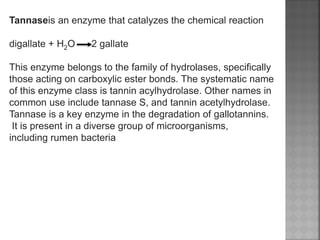
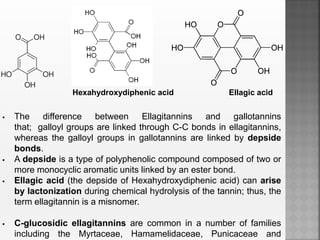
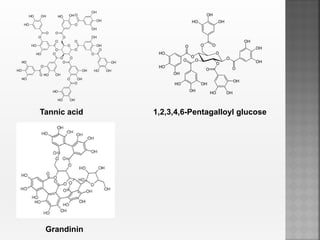
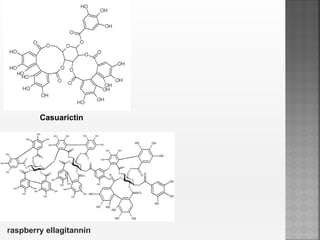
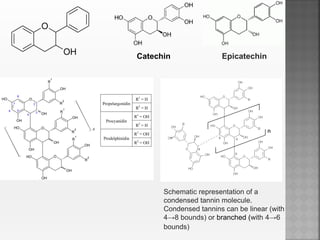
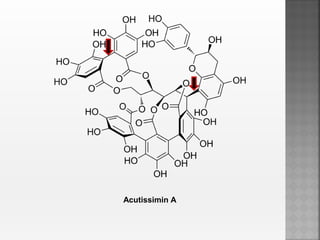
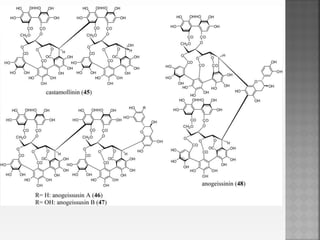
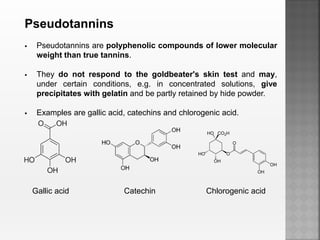
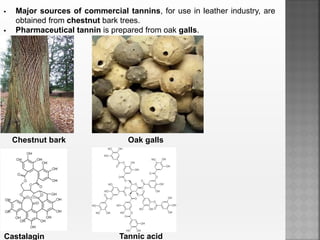
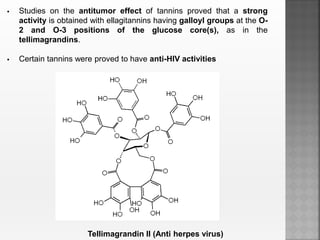
Tannins are high molecular weight phenolic compounds that can precipitate proteins. They are classified as hydrolysable tannins, condensed tannins, and complex tannins. Tannins are found in plants and have properties such as astringency. They have traditional medical uses as styptics and protectants. Tannins also have economic importance in industries like leather production and ink manufacturing.