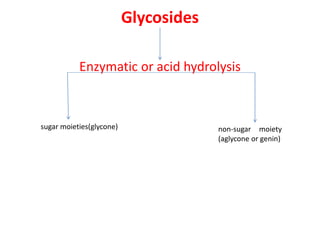
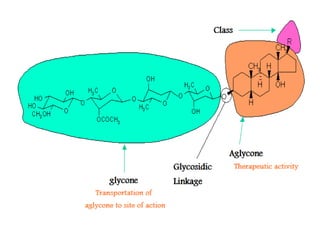
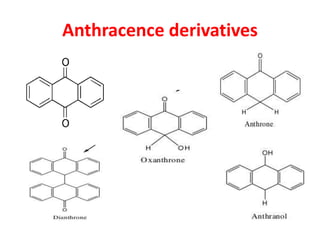
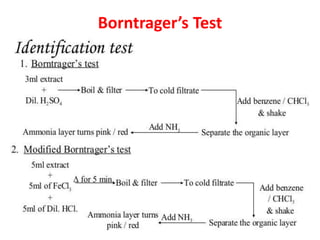
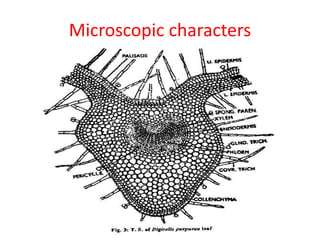
1. Glycosides are organic compounds found in plants and animals that contain a sugar (glycone) and non-sugar (aglycone or genin) portion. Upon hydrolysis, the sugar and non-sugar portions separate.
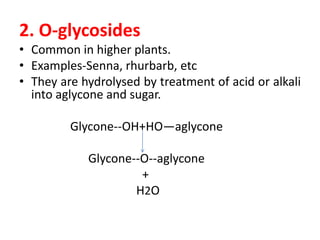
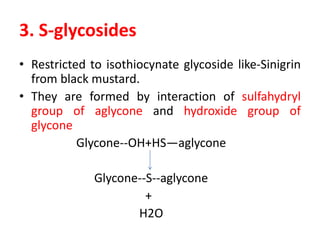
2. There are several types of glycosides based on the atom involved in the glycosidic linkage between the glycone and aglycone, including O-, C-, S-, and N-glycosides.
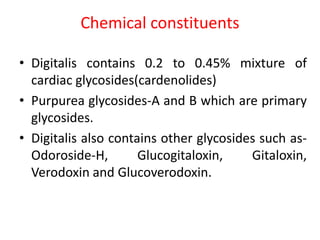
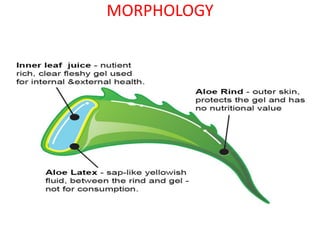
3. Two common cardiac glycoside drugs that contain glycosides are Digitalis and Aloe. Digitalis contains compounds like digitoxin and gitoxin that have cardiac effects. Aloe contains compounds like aloin that have laxative effects.