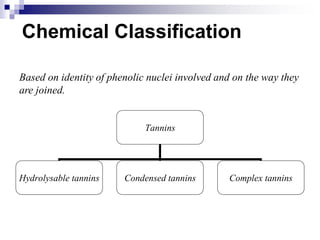
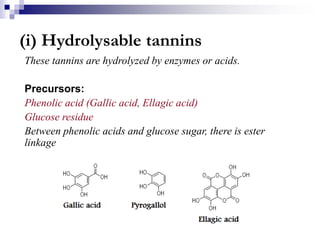
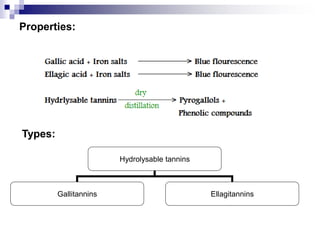
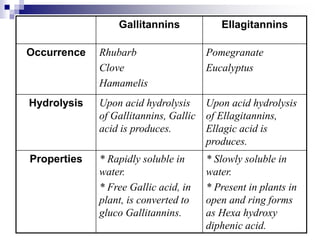
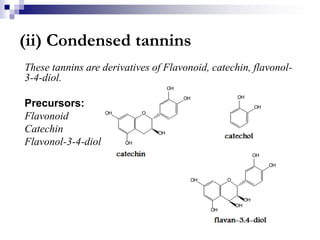
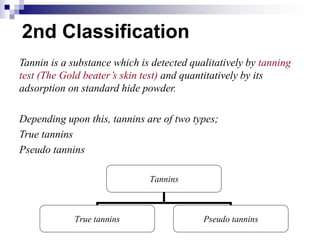
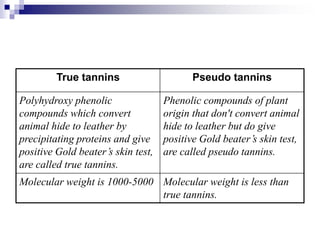
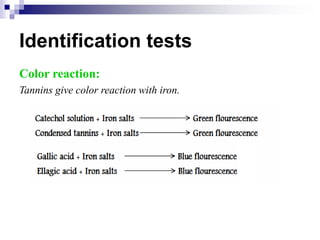
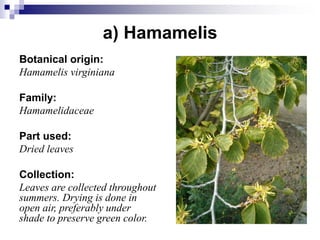
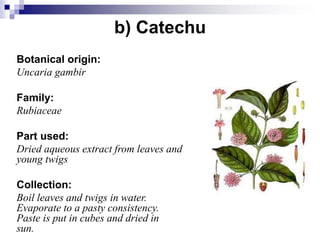
Tannins are polyphenolic compounds that occur naturally in many plant species. They are classified as hydrolysable tannins, condensed tannins, or complex tannins depending on their chemical structure. Tannins can be identified through tests like the goldbeater's skin test where they cause animal skins to darken, and the matchstick test where they turn matchsticks pink or red. Examples of tannin-containing plants described in the document include hamamelis, catechu, and nut galls.