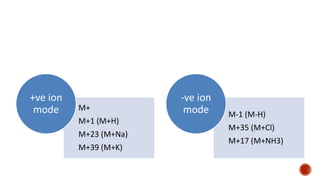
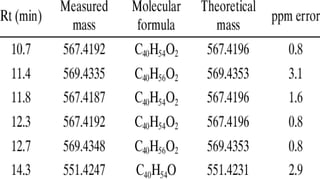
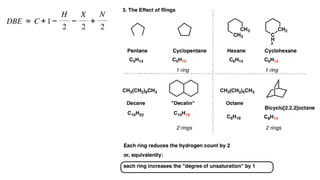
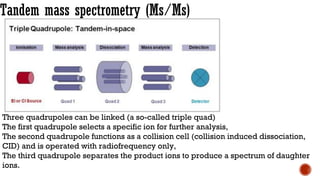
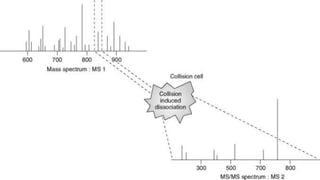
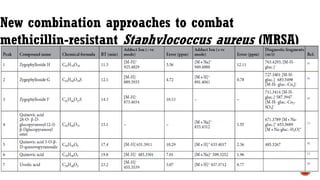
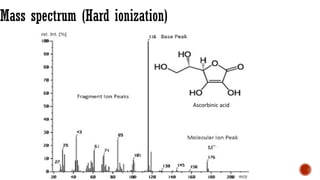
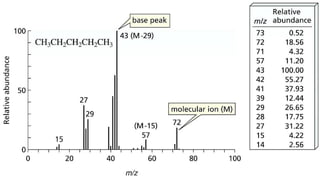
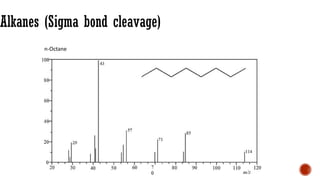
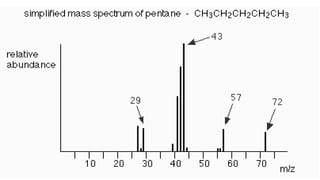
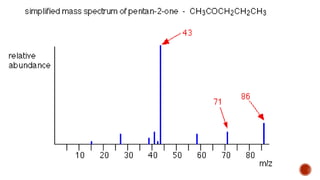
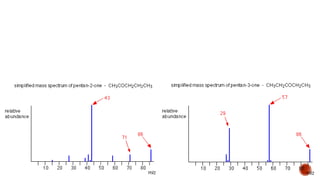
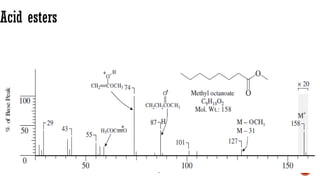
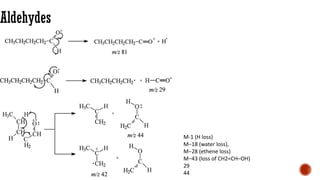
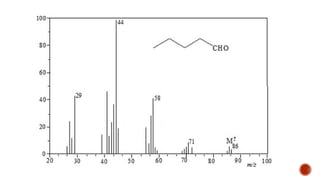
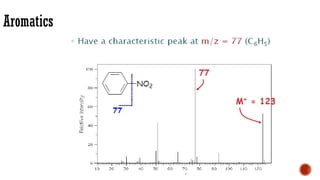
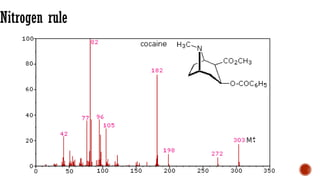
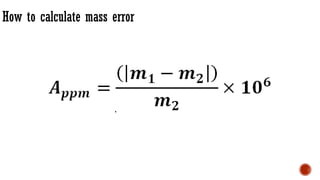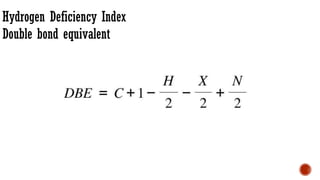The document presents an overview of mass spectrometry applications, detailing hard ionization techniques, high resolution mass spectrometry (HR-MS), and the interpretation of isotope patterns in mass spectra. It explains how mass spectrometers separate ions and determine molecular formulas, emphasizing the significance of isotope abundance, particularly in compounds containing chlorine and bromine. The author, Dr. Ahmed Metwaly, is an associate professor at Al-Azhar University and discusses methodologies for combatting methicillin-resistant Staphylococcus aureus (MRSA) in relation to mass spectrometry.

![Ethers (inductive cleavage)
Acylium ions, [RCO]+](https://image.slidesharecdn.com/mass2021-4applications-210419211505/85/Mass-2021-4-applications-7-320.jpg)
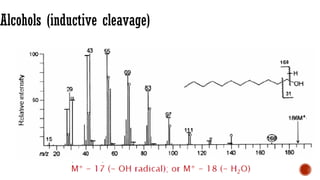
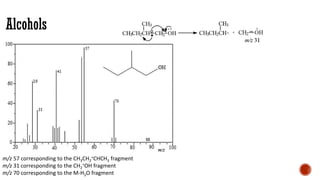
![Acids [McLafferty rearrangement]
C
O
H2C OH
R CH
H
CH2
-e-
C
O
H2C OH
R CH
H
CH2
rH
C
O
H2C OH
R CH
CH2
H
C
O
H2C OH
R CH
CH2
H
+
m/z = 60
](https://image.slidesharecdn.com/mass2021-4applications-210419211505/85/Mass-2021-4-applications-12-320.jpg)
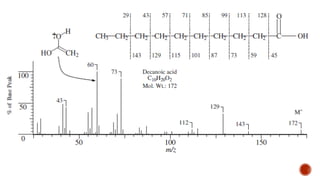
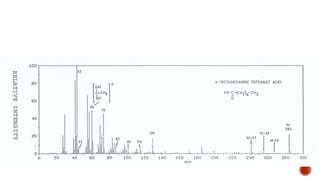
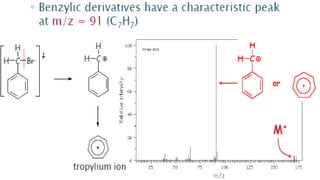
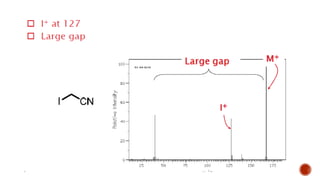

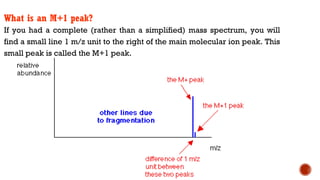
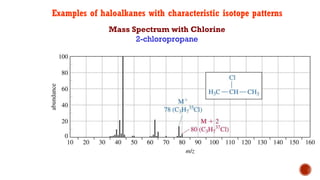
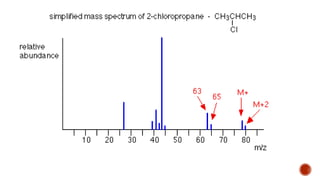
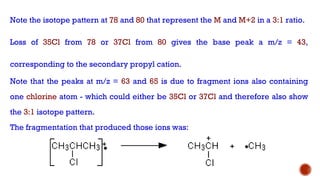
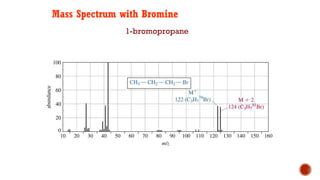
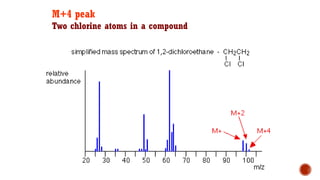
![This is most apparent when atoms such as bromine or chlorine are present.
Peaks at "M" and "M+2" are obtained.
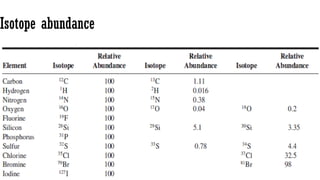
Bromine isotopes [79Br : 81Br] have the same abundance intensity (having M and M+2 in
ratio 1:1).
Chlorine [35Cl : 37Cl] have difference in the abundance, so the intensity of M and M+2 is
in ratio 3:1.
The intensity ratios in the isotope patterns are due to the natural abundance of the
isotopes.
Therefore, differentiation between the mass spectra of chlorine- and bromine-containing
compounds is possible.
M+2 peak](https://image.slidesharecdn.com/mass2021-4applications-210419211505/85/Mass-2021-4-applications-26-320.jpg)