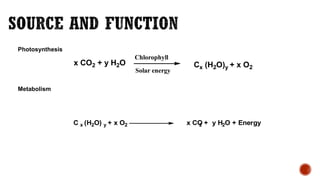
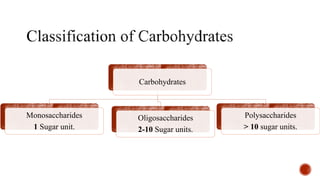
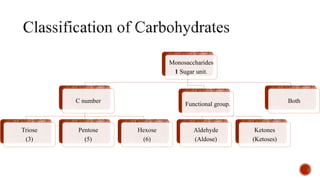
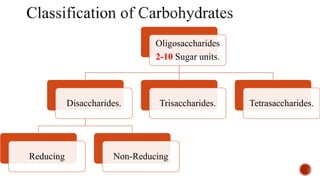
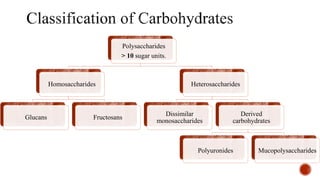
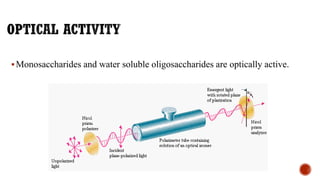
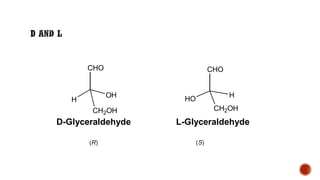
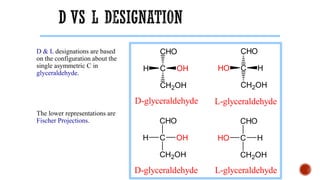
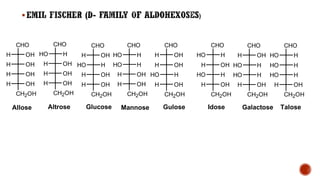
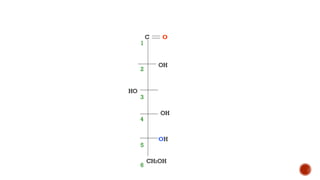
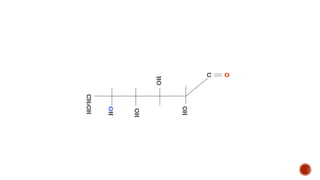
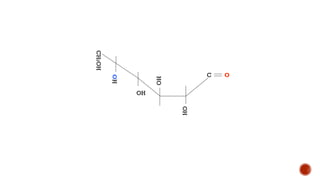
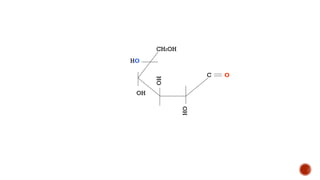
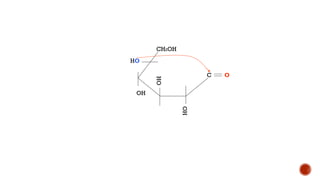
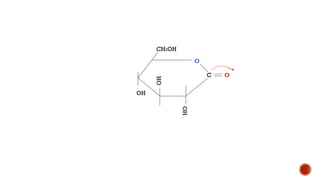
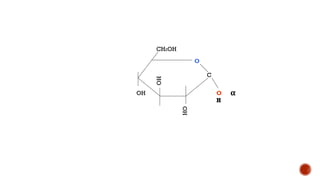
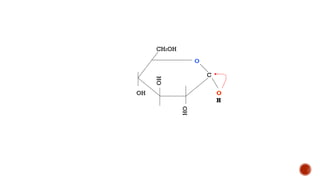
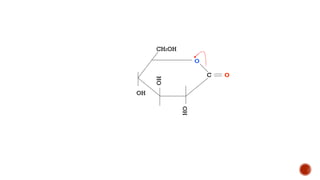
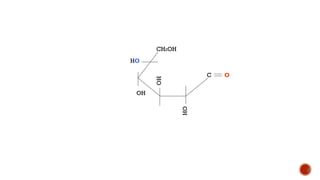
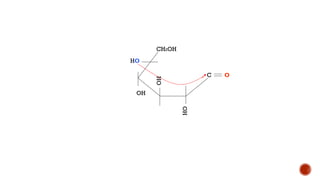
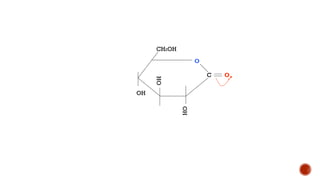
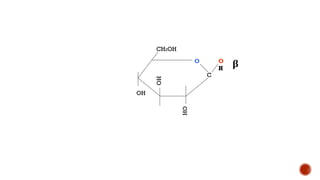
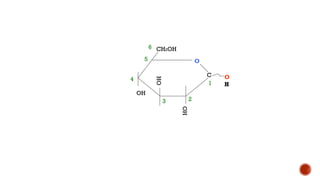
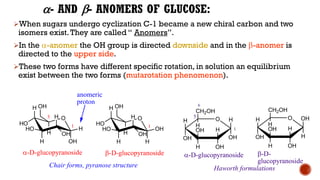
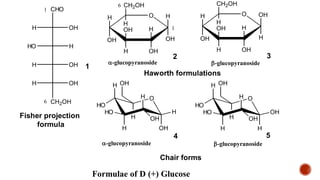
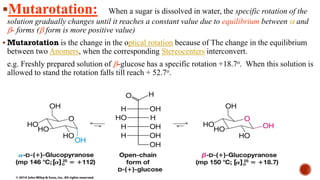
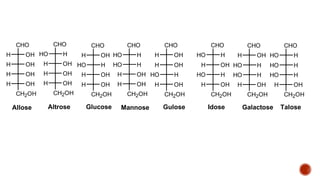
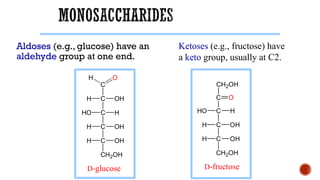
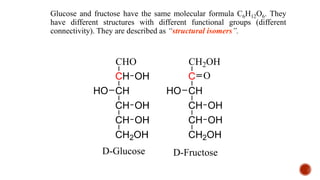
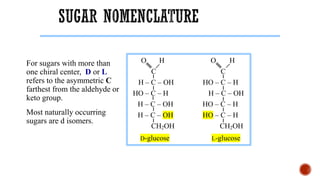
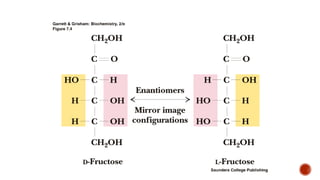
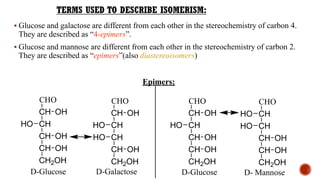
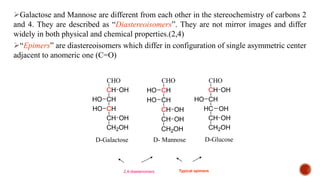
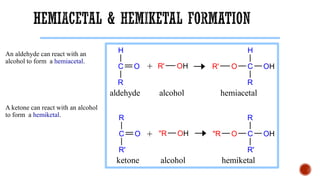
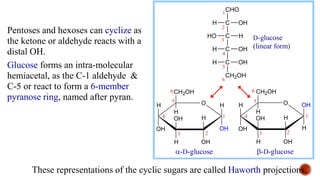
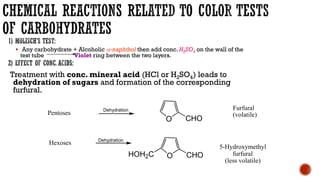
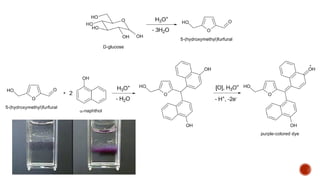
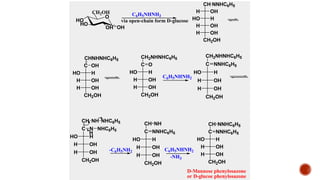
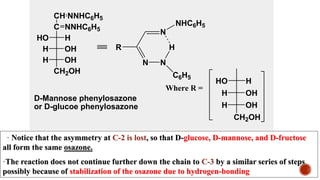
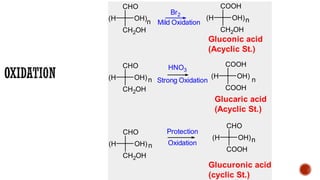
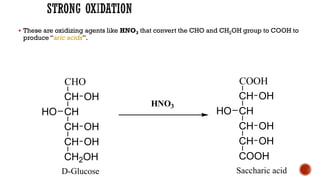
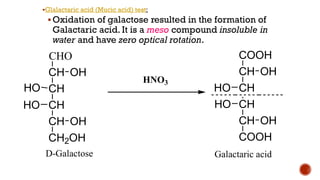
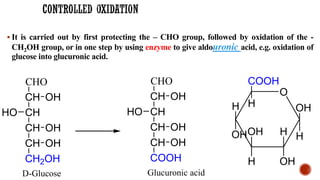
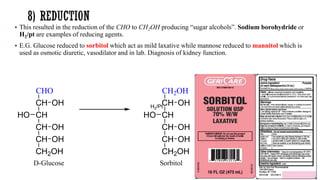
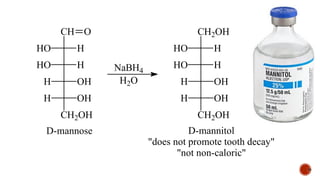
This document provides information about Ahmed Metwaly's academic positions and qualifications. It then discusses carbohydrates, including their definition, classification into mono-, oligo-, and polysaccharides, and the structures of common monosaccharides like glucose and fructose. The rest of the document covers carbohydrate chemistry, focusing on structural isomers of monosaccharides, cyclic forms, mutarotation, and reactions like those involving furfural.