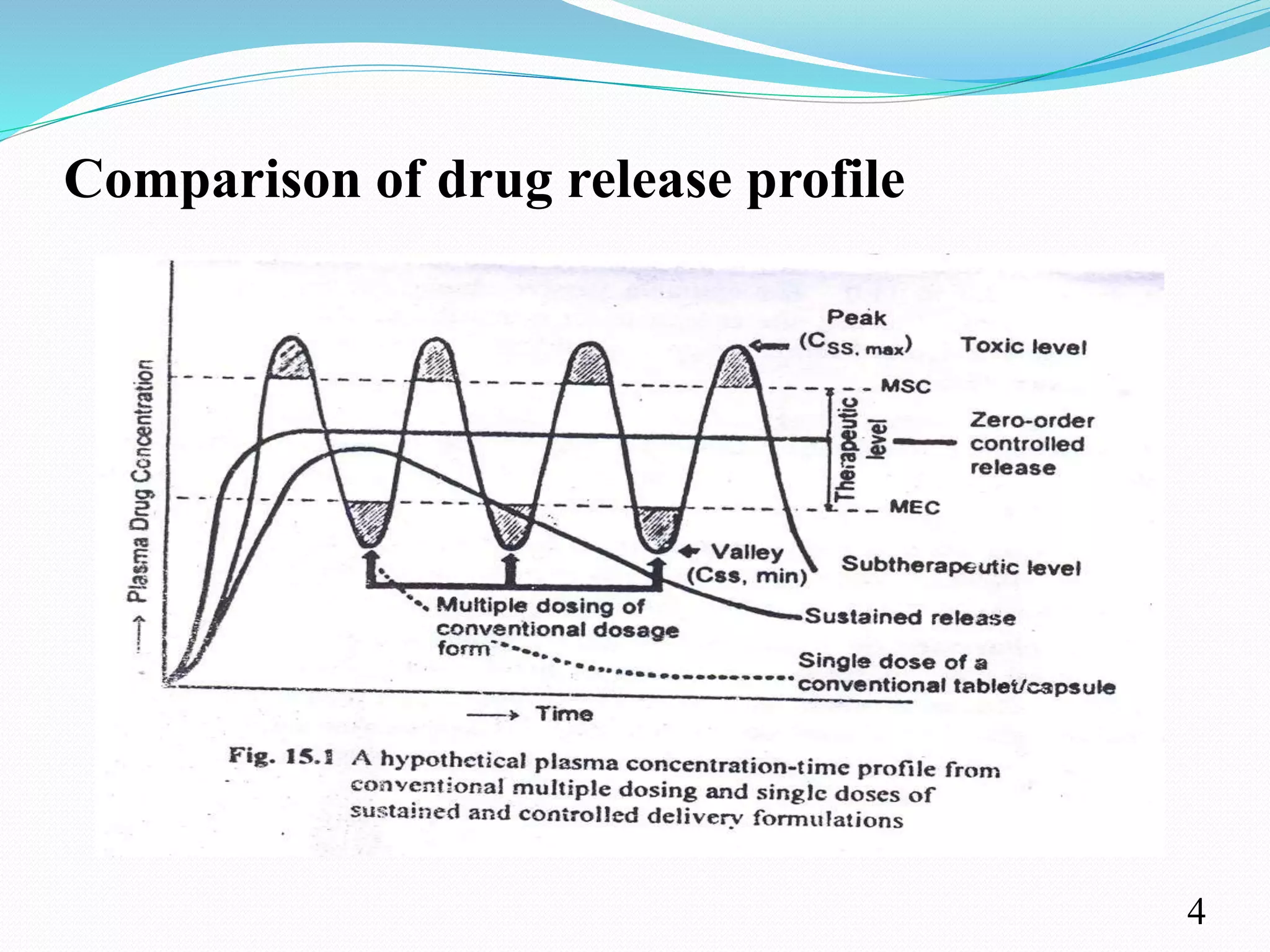
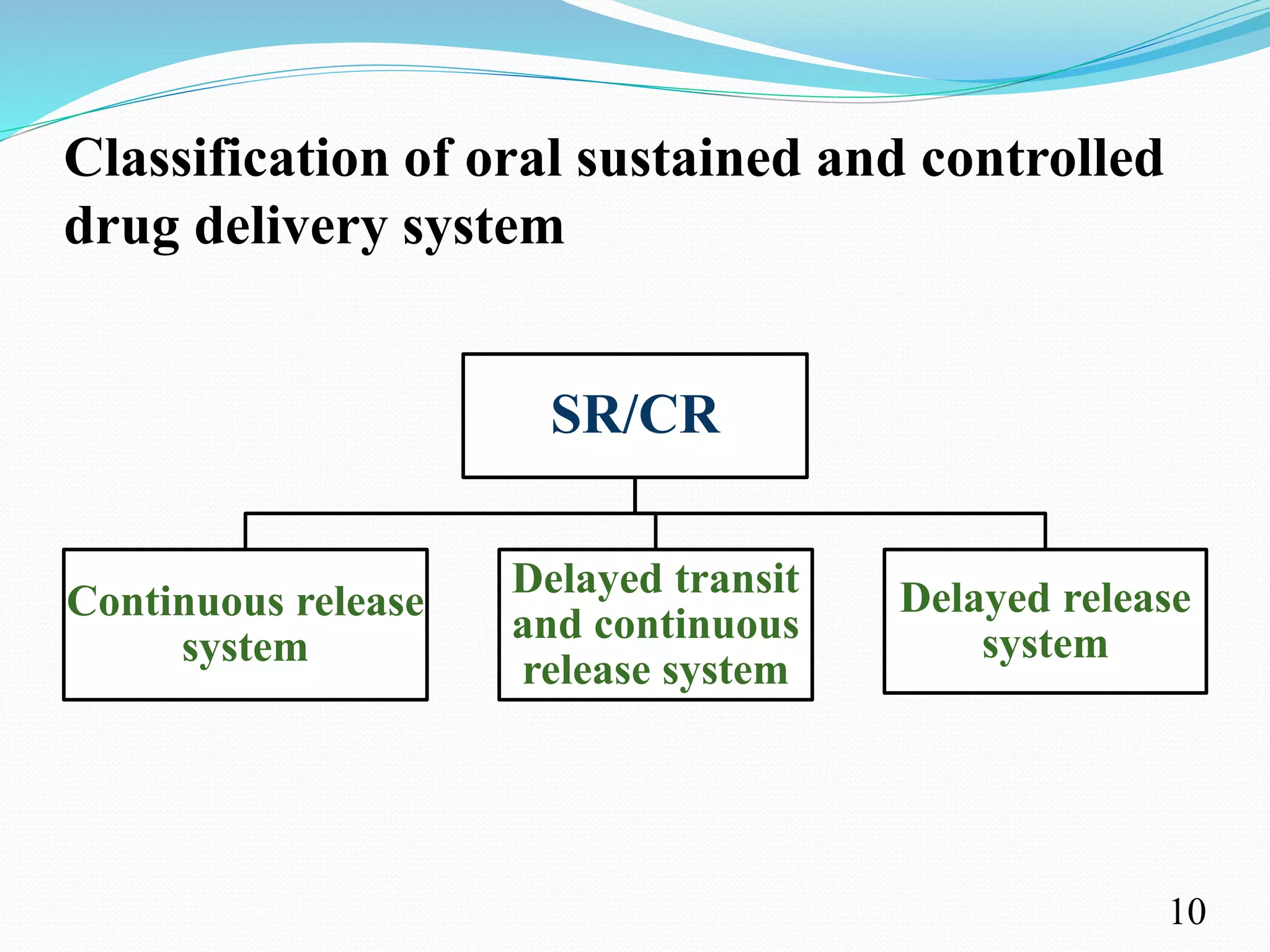
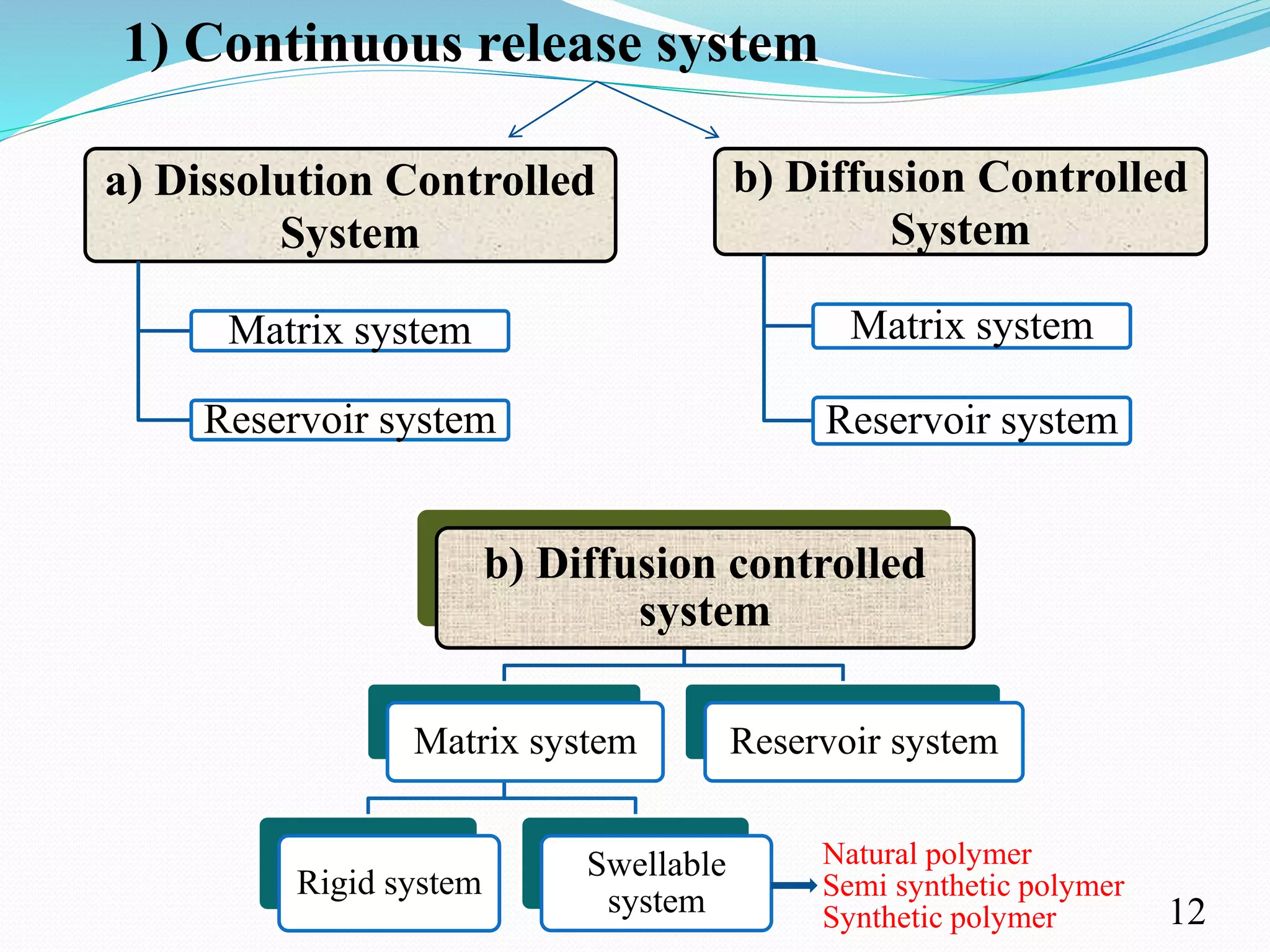
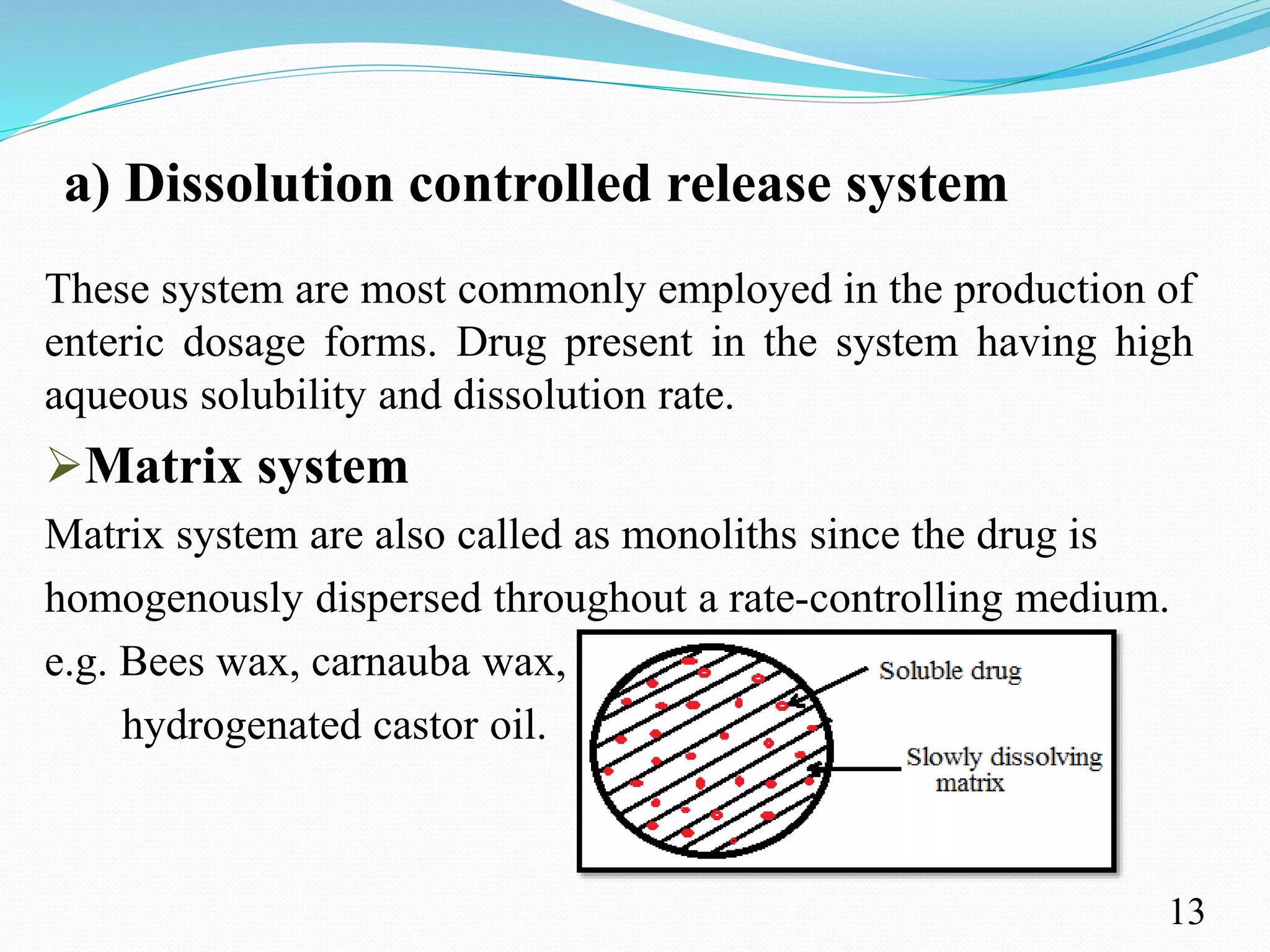
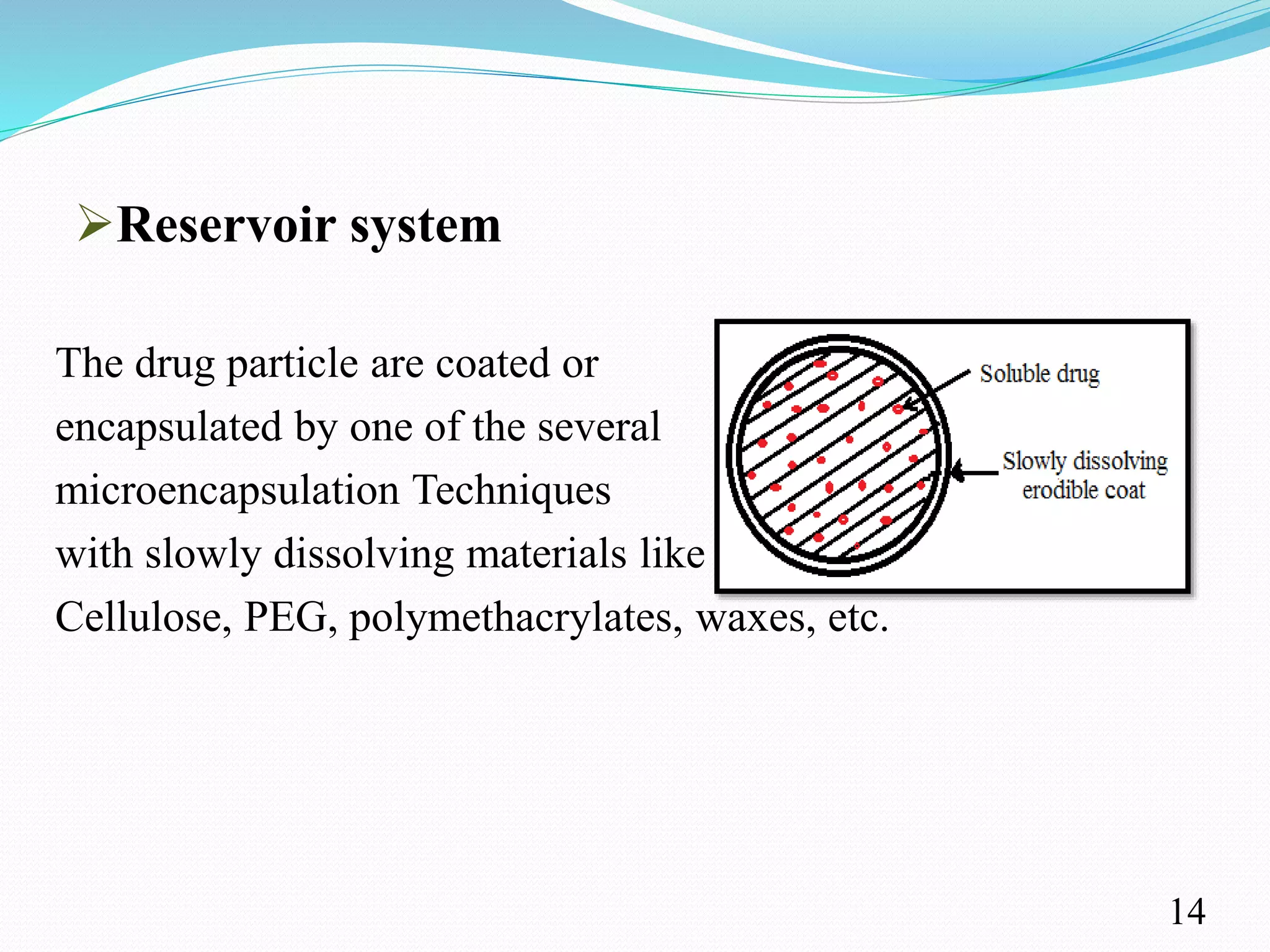
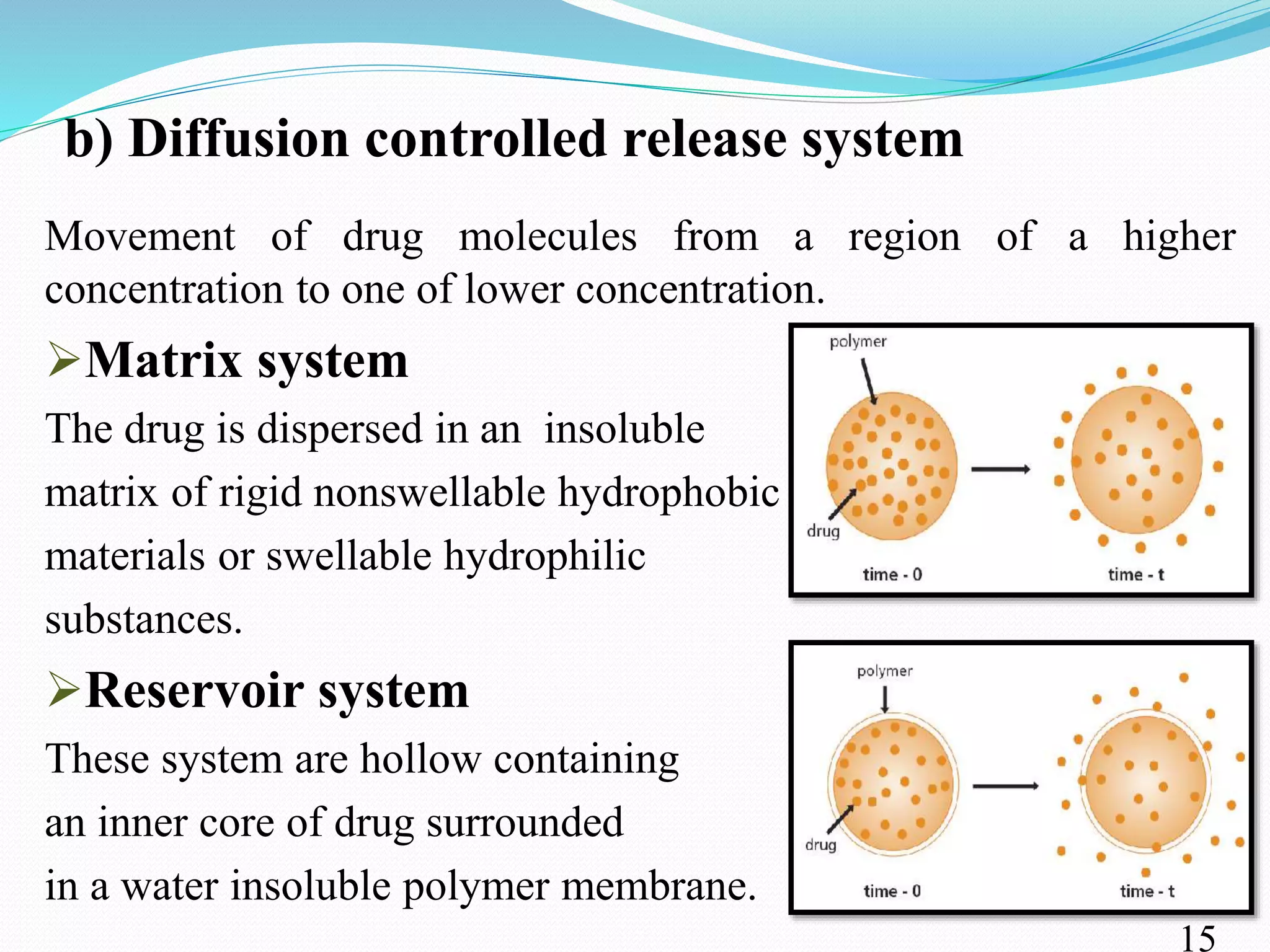
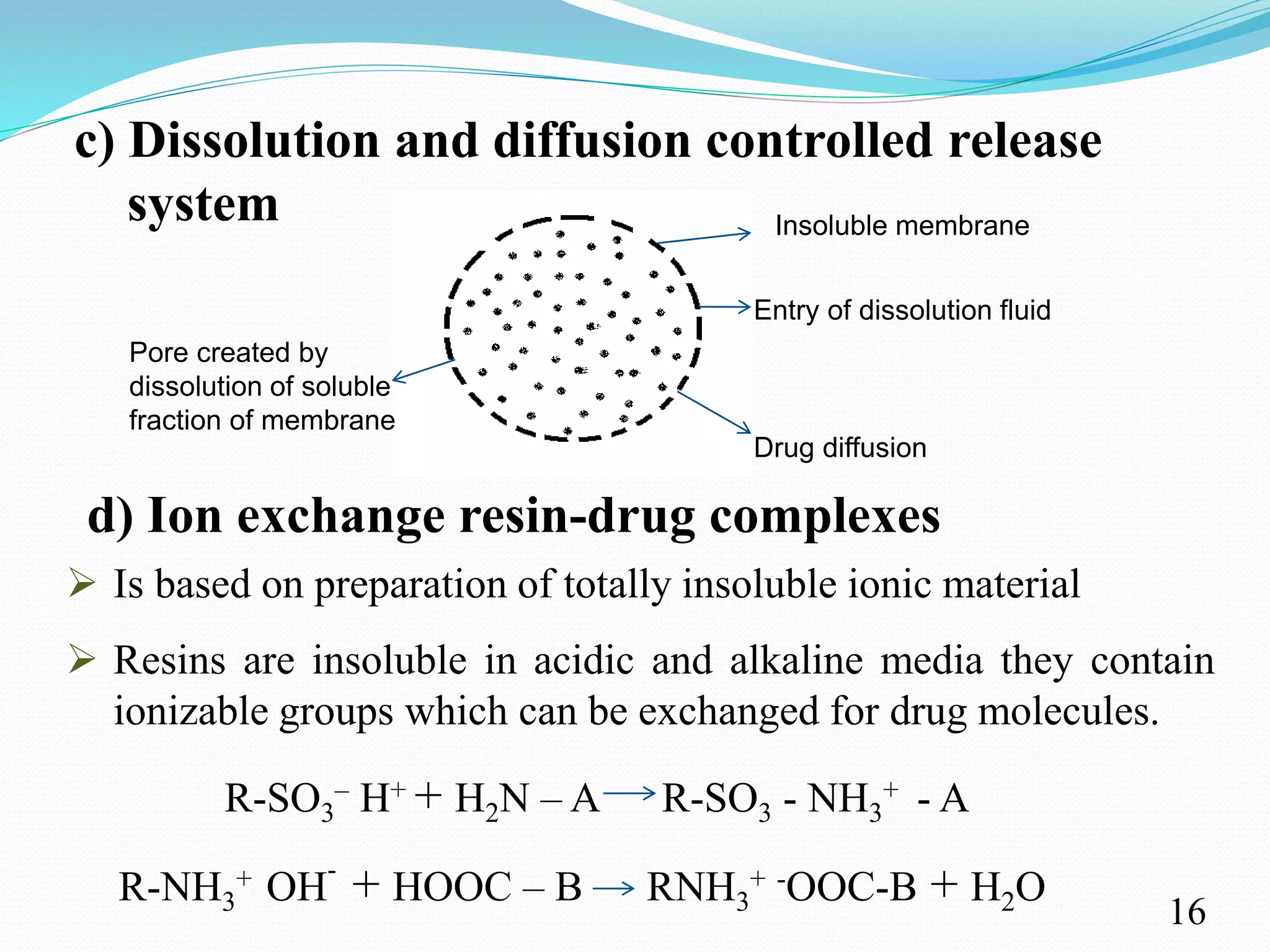
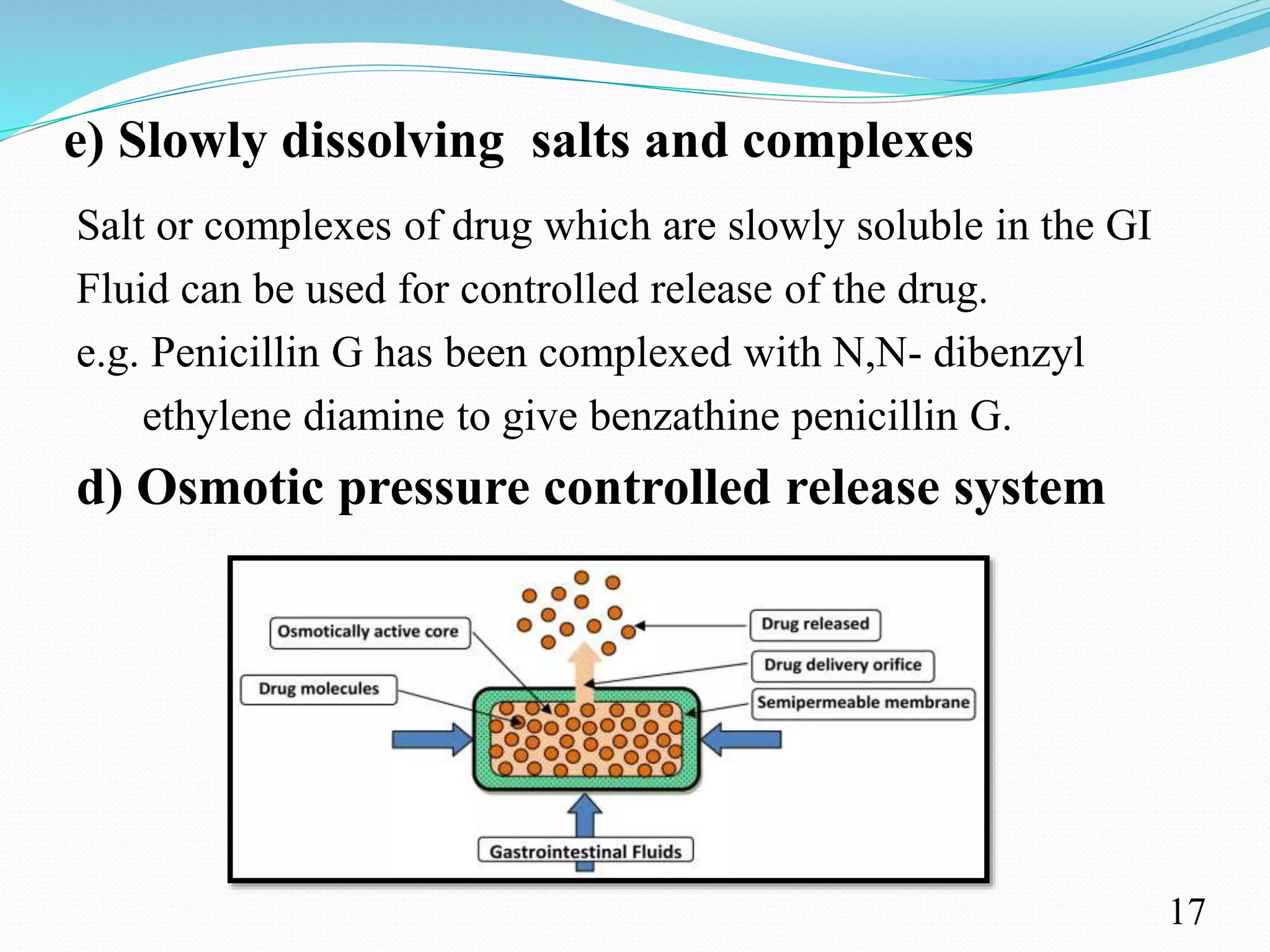
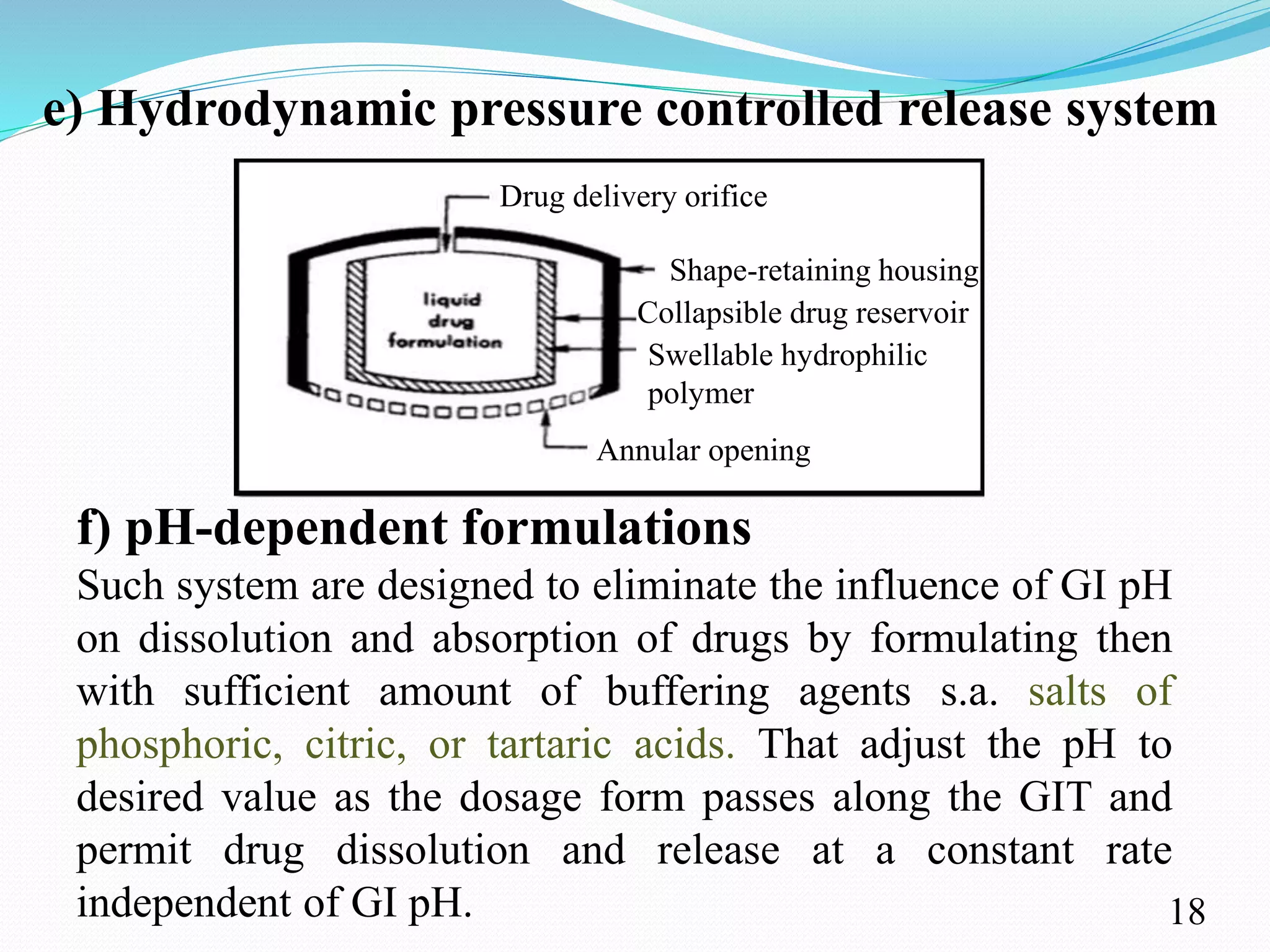
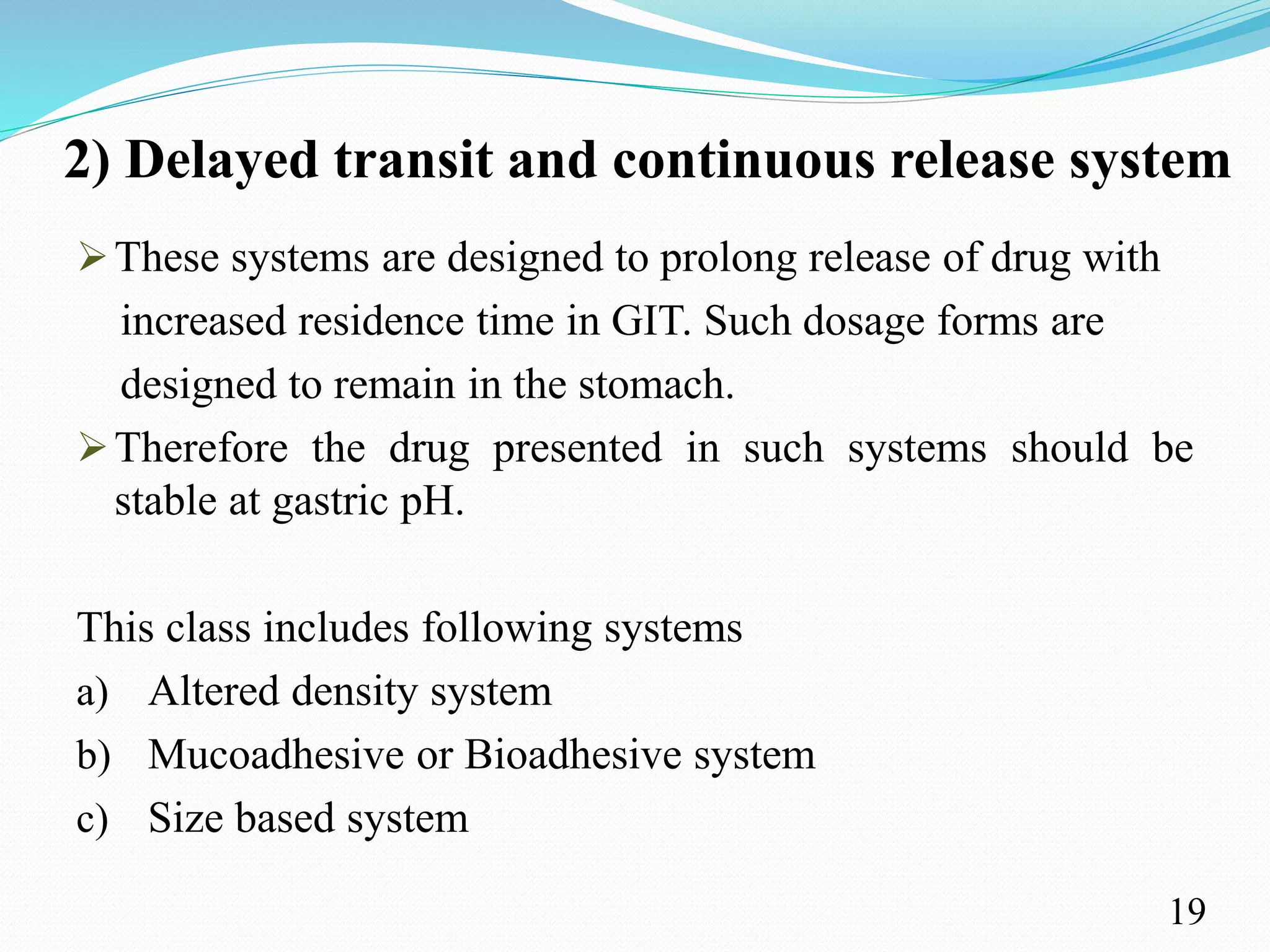
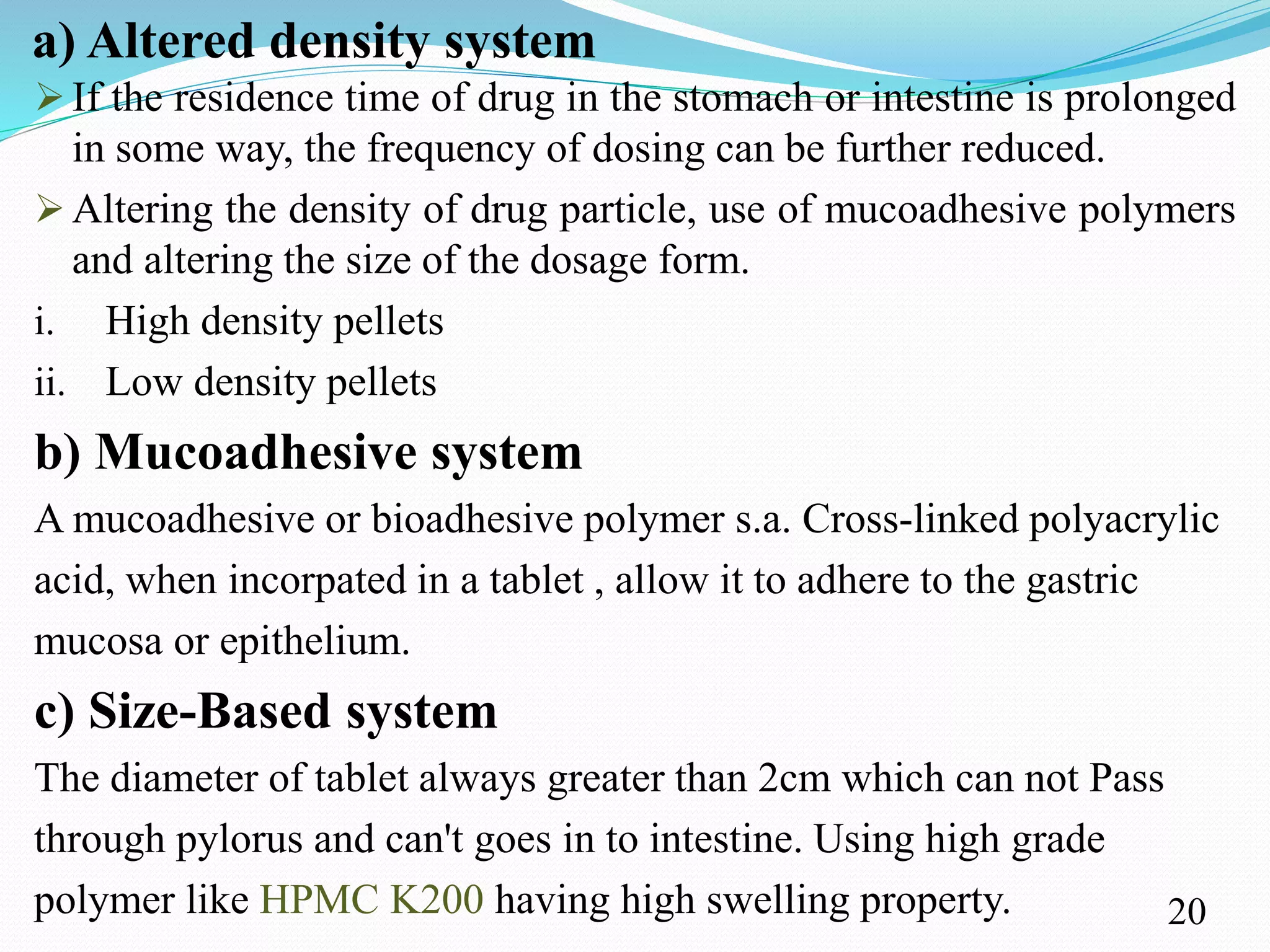
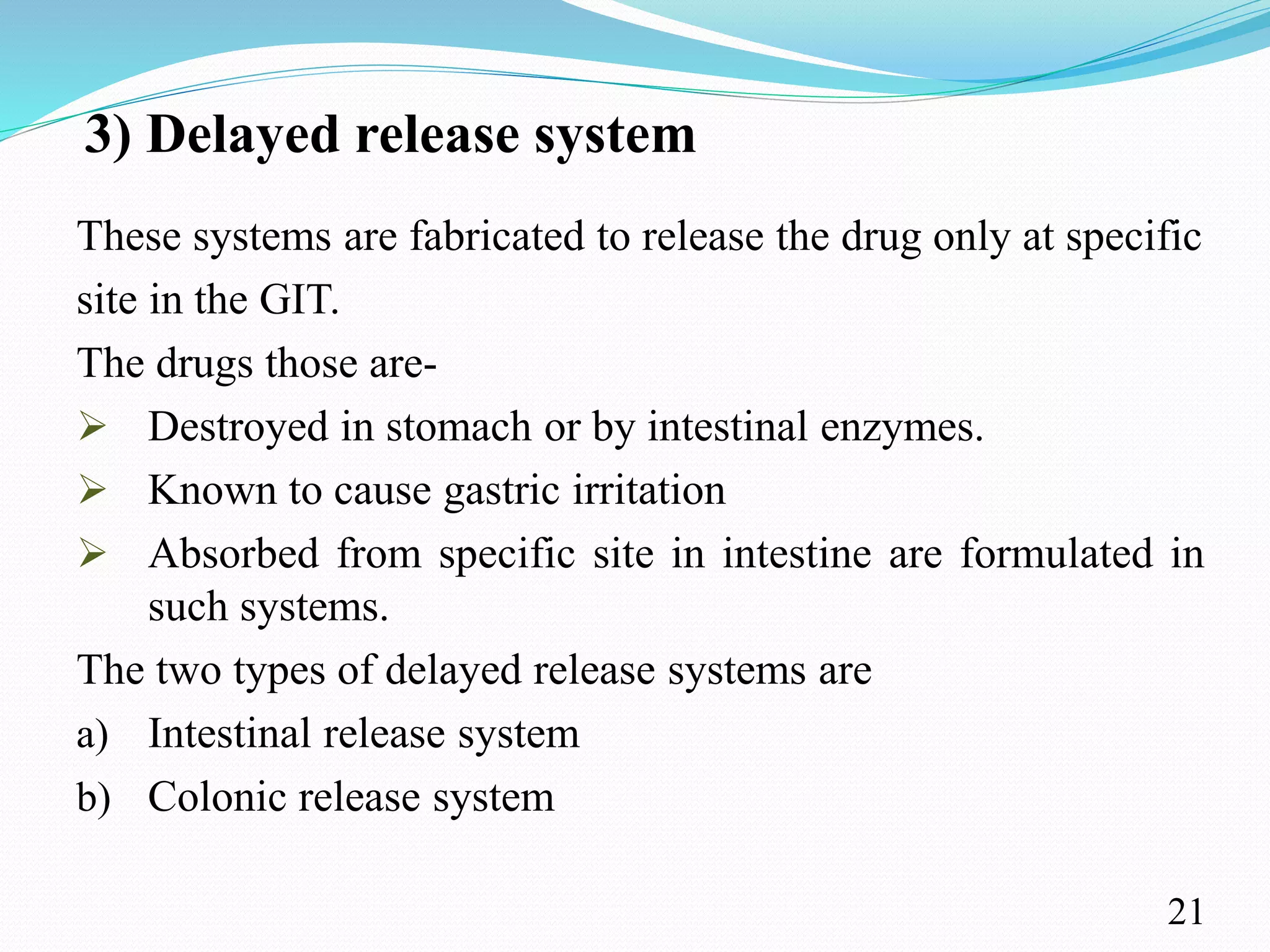
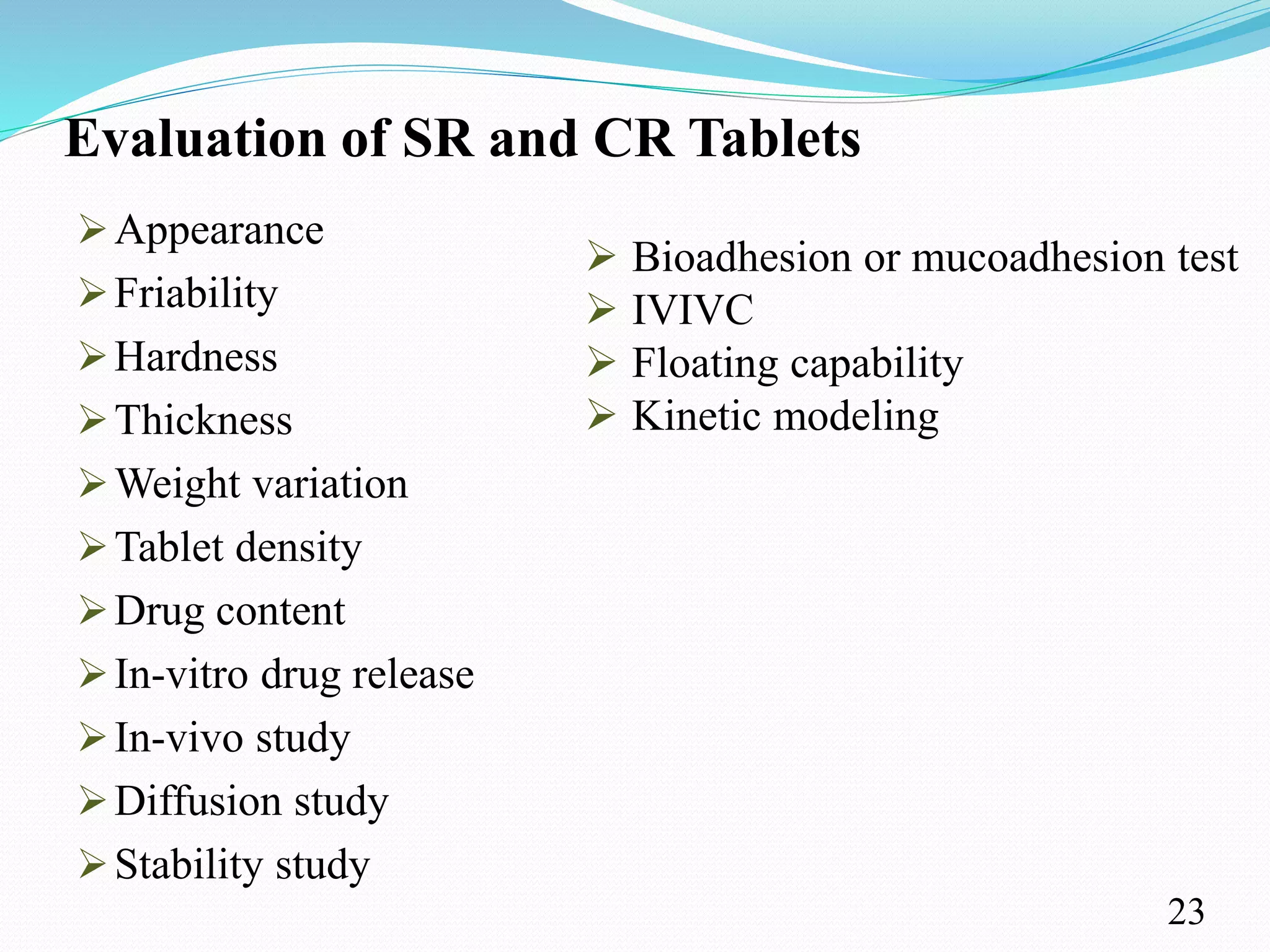
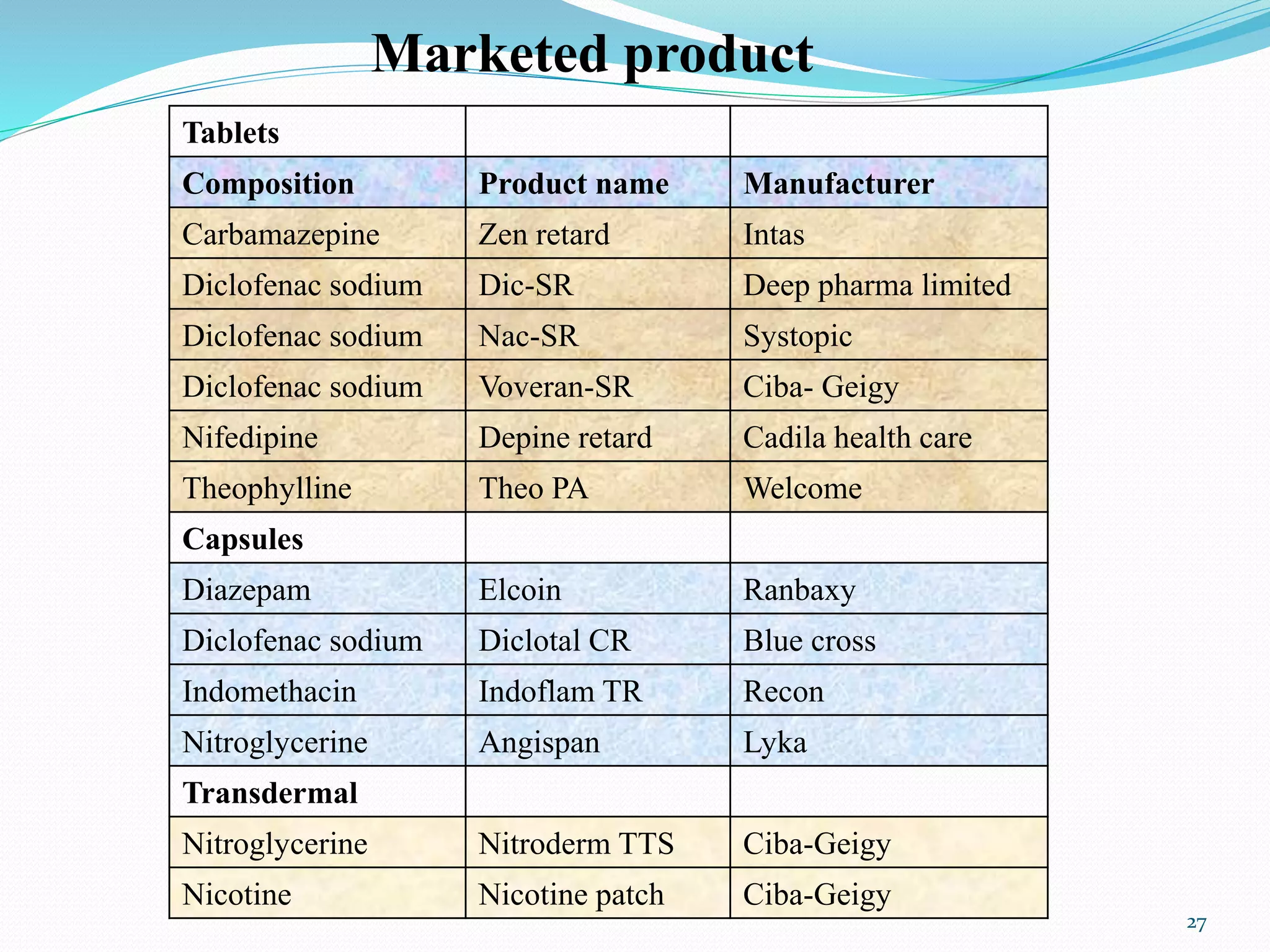
This document provides an overview of sustained and controlled drug delivery systems (SR and CRDDS). It defines SR and CRDDS and compares their drug release profiles. The advantages include improved bioavailability and compliance while disadvantages include dose dumping and adjustment difficulties. Drugs are selected based on their physicochemical, pharmacokinetic, and pharmacodynamic properties. SR and CRDDS are classified into continuous release, delayed transit-continuous release, and delayed release systems. They are evaluated for properties like drug release and stability. Applications include oral, ocular, transdermal, and colonic delivery. Marketed products of these systems in tablets, capsules, and transdermal forms are also mentioned.