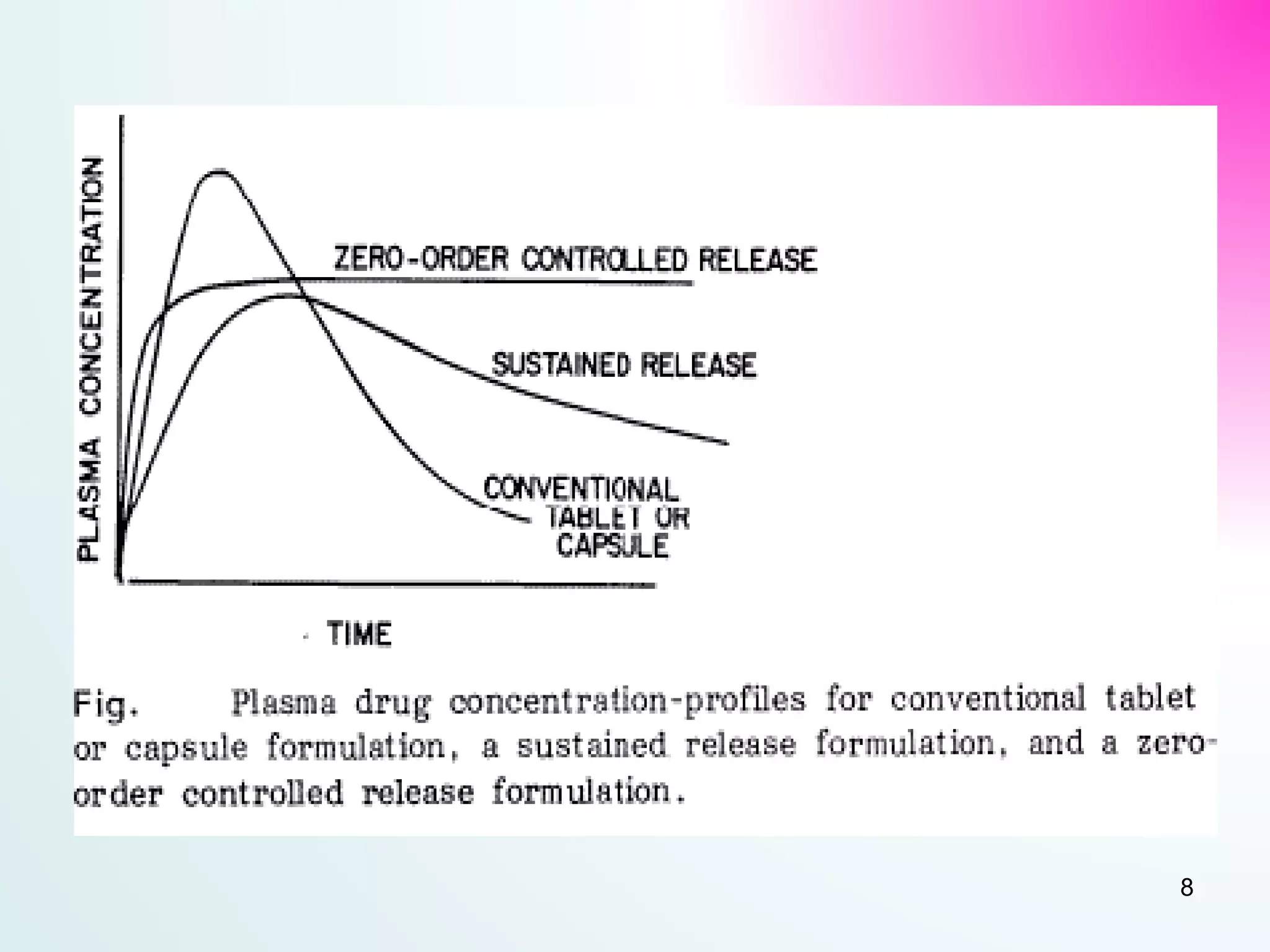
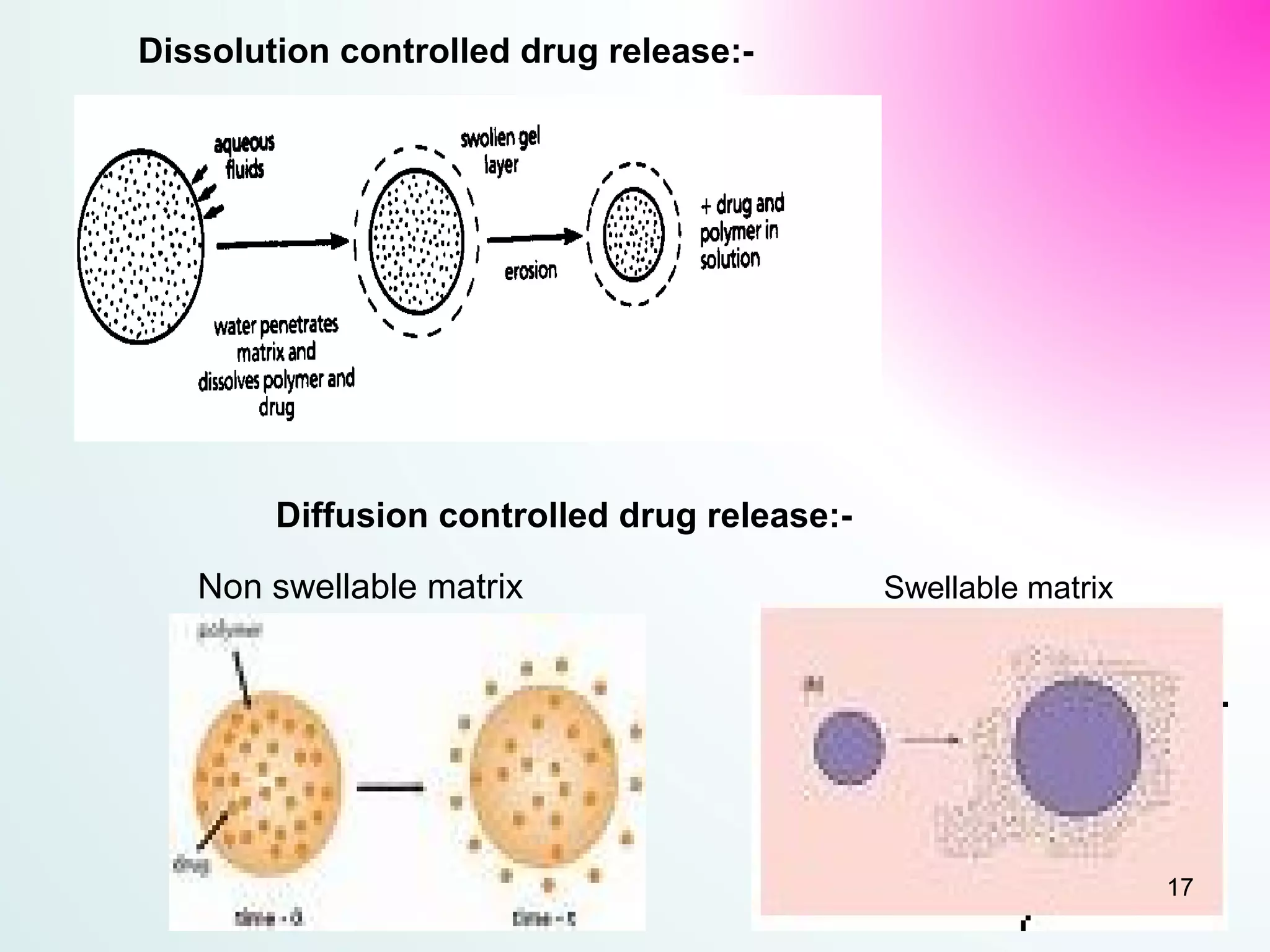
The document discusses sustained release dosage forms (SRDF), which are designed to provide prolonged therapeutic effect by releasing medication over an extended period after a single dose. It covers various drug release types, suitable drug properties, and the use of various polymers for controlled release mechanisms, along with their advantages and disadvantages. Additionally, it highlights factors affecting drug absorption and safety while providing references for further reading.
![Sustained Release Dosage
Form [SRDF]
1](https://image.slidesharecdn.com/sustainedreleasedosageformsrdf-160516130442/75/Sustained-release-dosage-form-srdf-1-2048.jpg)