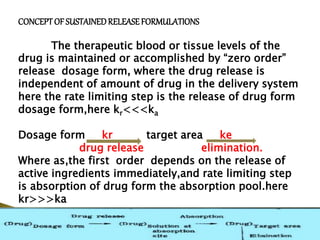
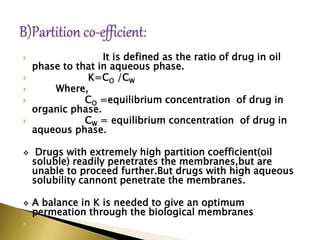
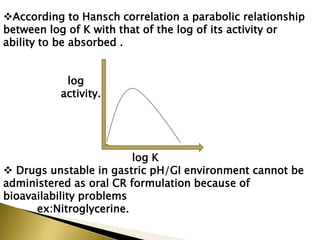
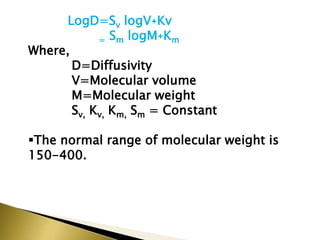
This document provides an introduction to sustained release and controlled release drug formulations. It defines sustained release as slowly releasing a drug over 8-12 hours, while controlled release delivers a drug at a predetermined rate for a specified time period. Some key advantages of these formulations are improved patient compliance, better drug utilization, and decreased side effects. Physicochemical drug properties like solubility, permeability and stability can impact whether a drug is suitable for these delivery systems. The document discusses various approaches for sustained and controlled release based on these physicochemical factors.