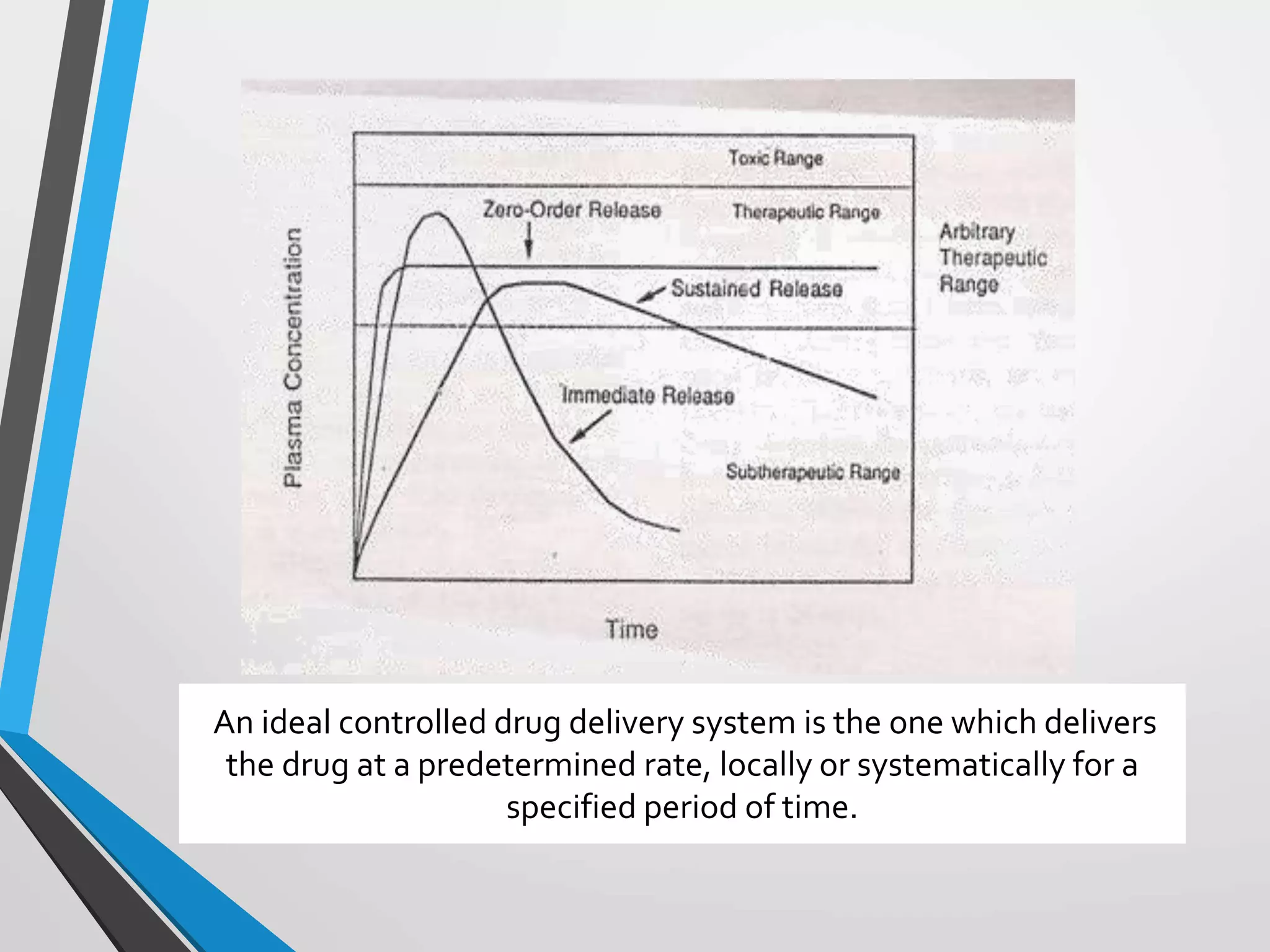
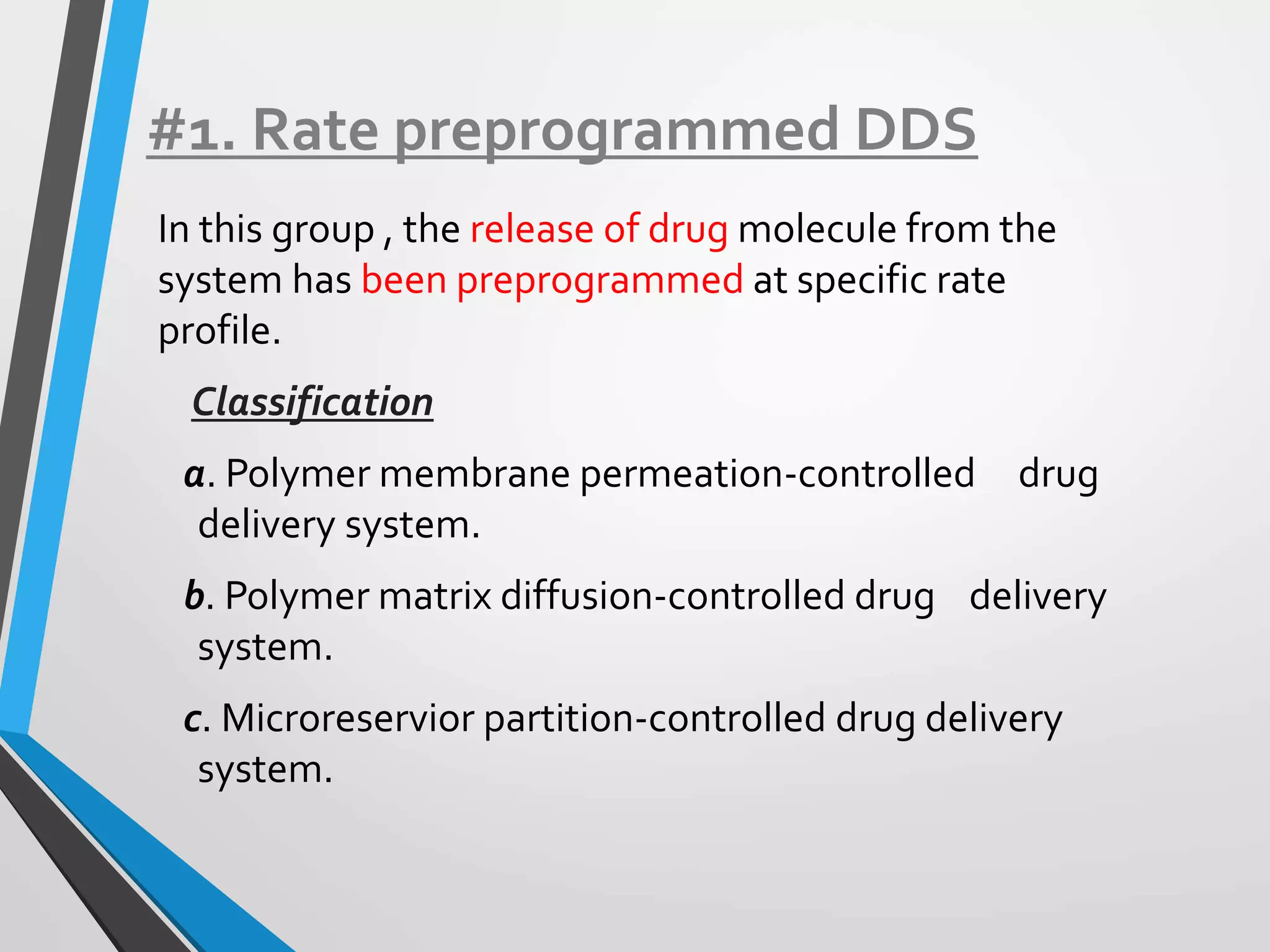
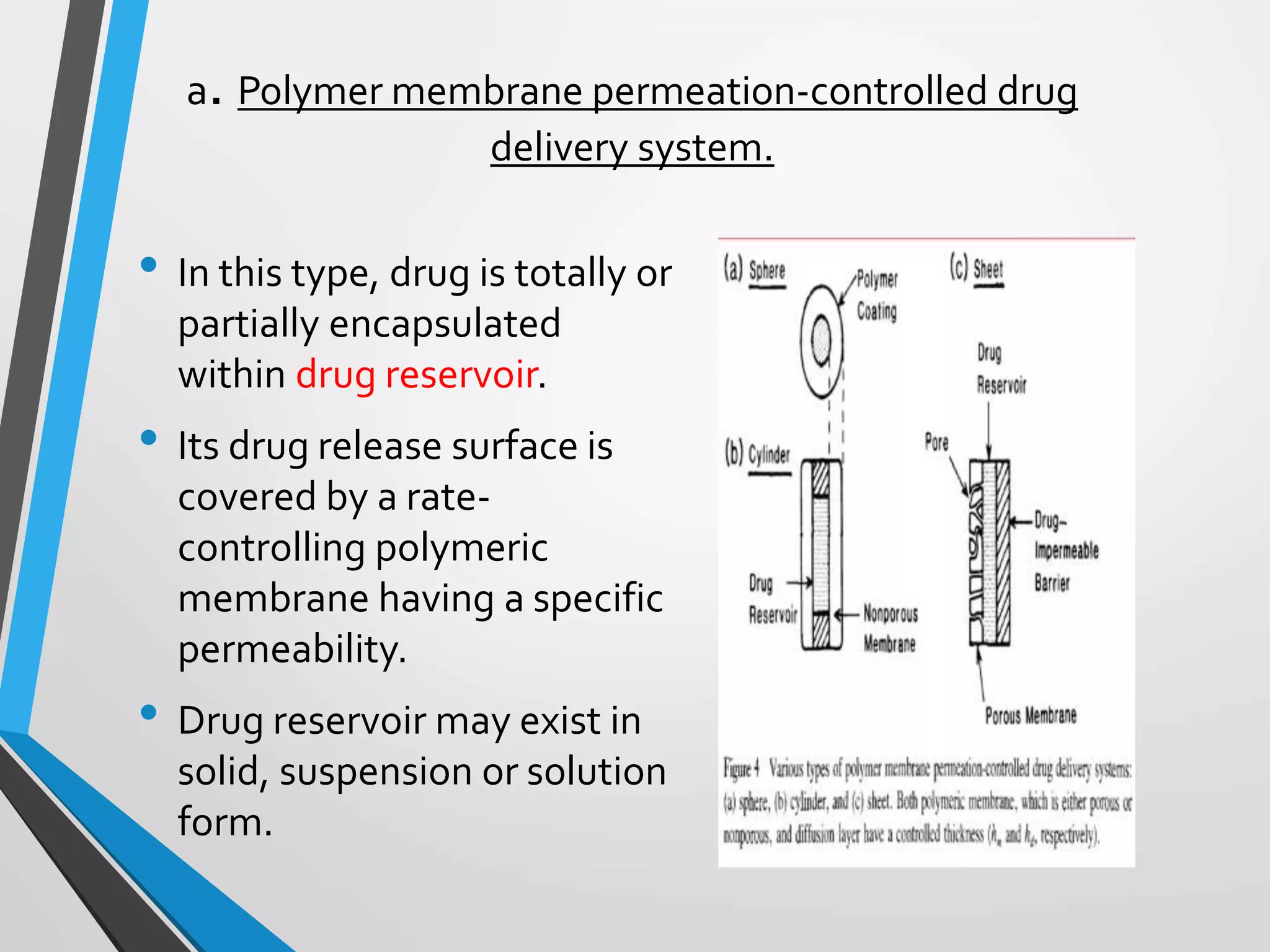
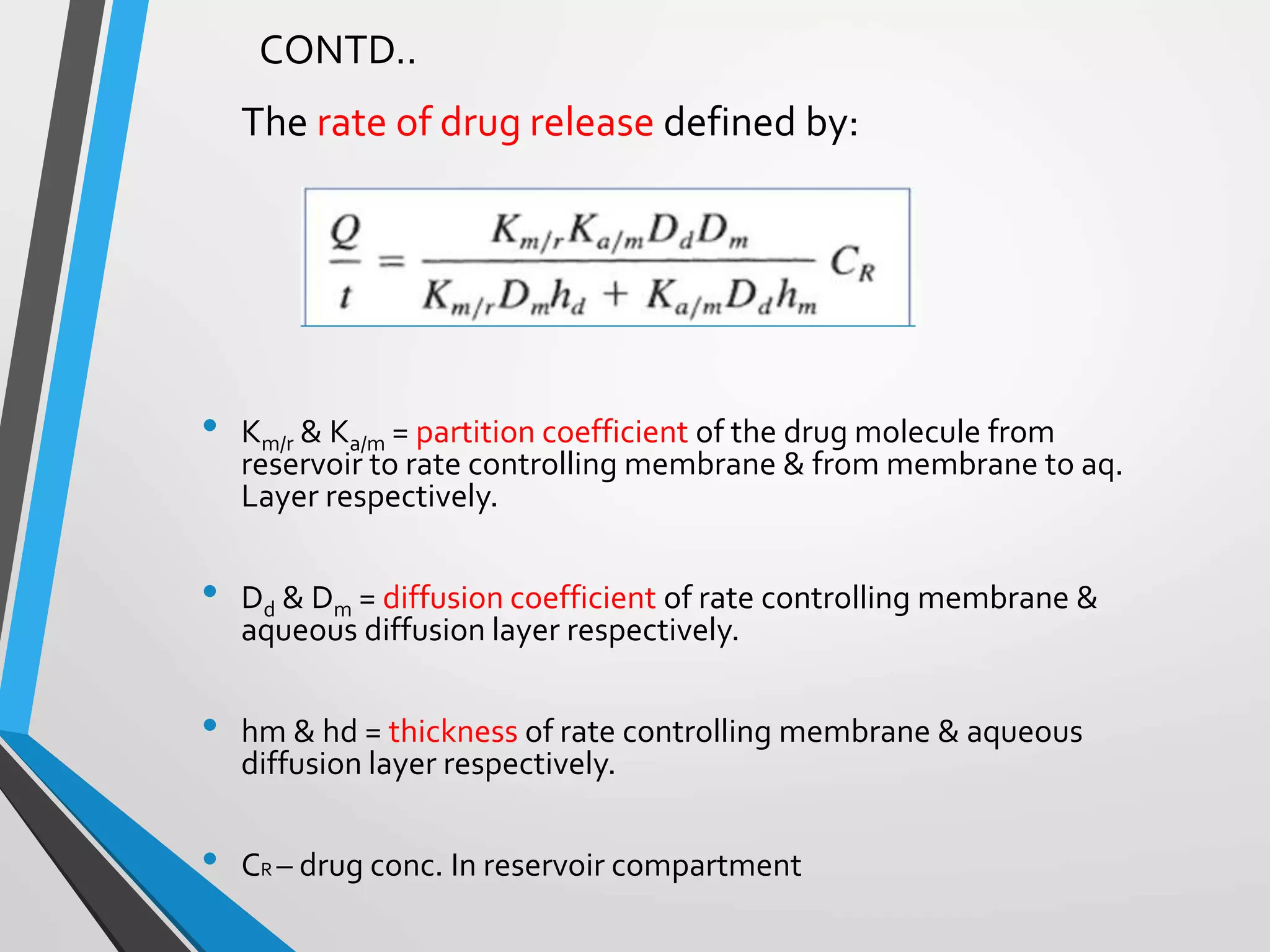
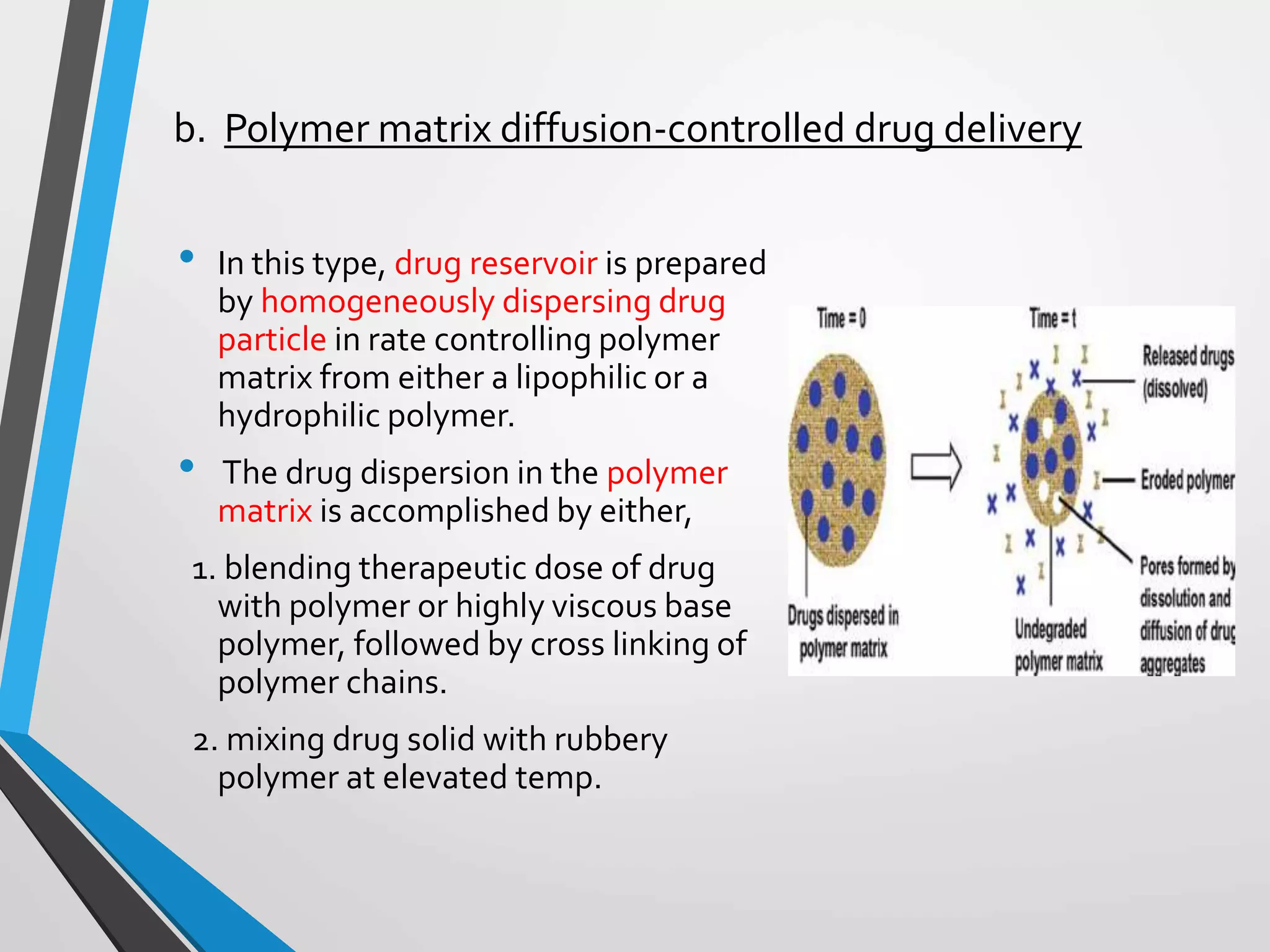
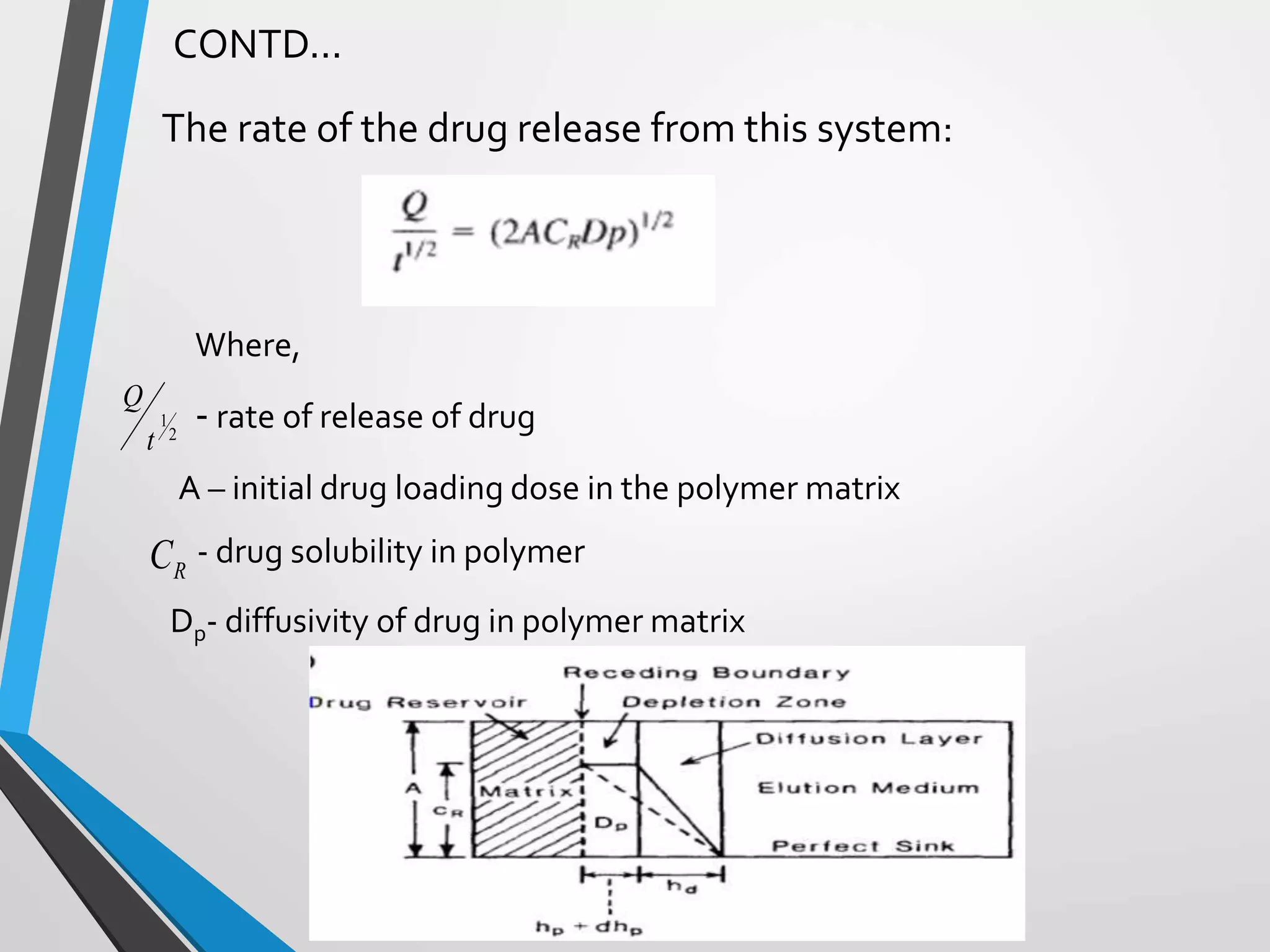
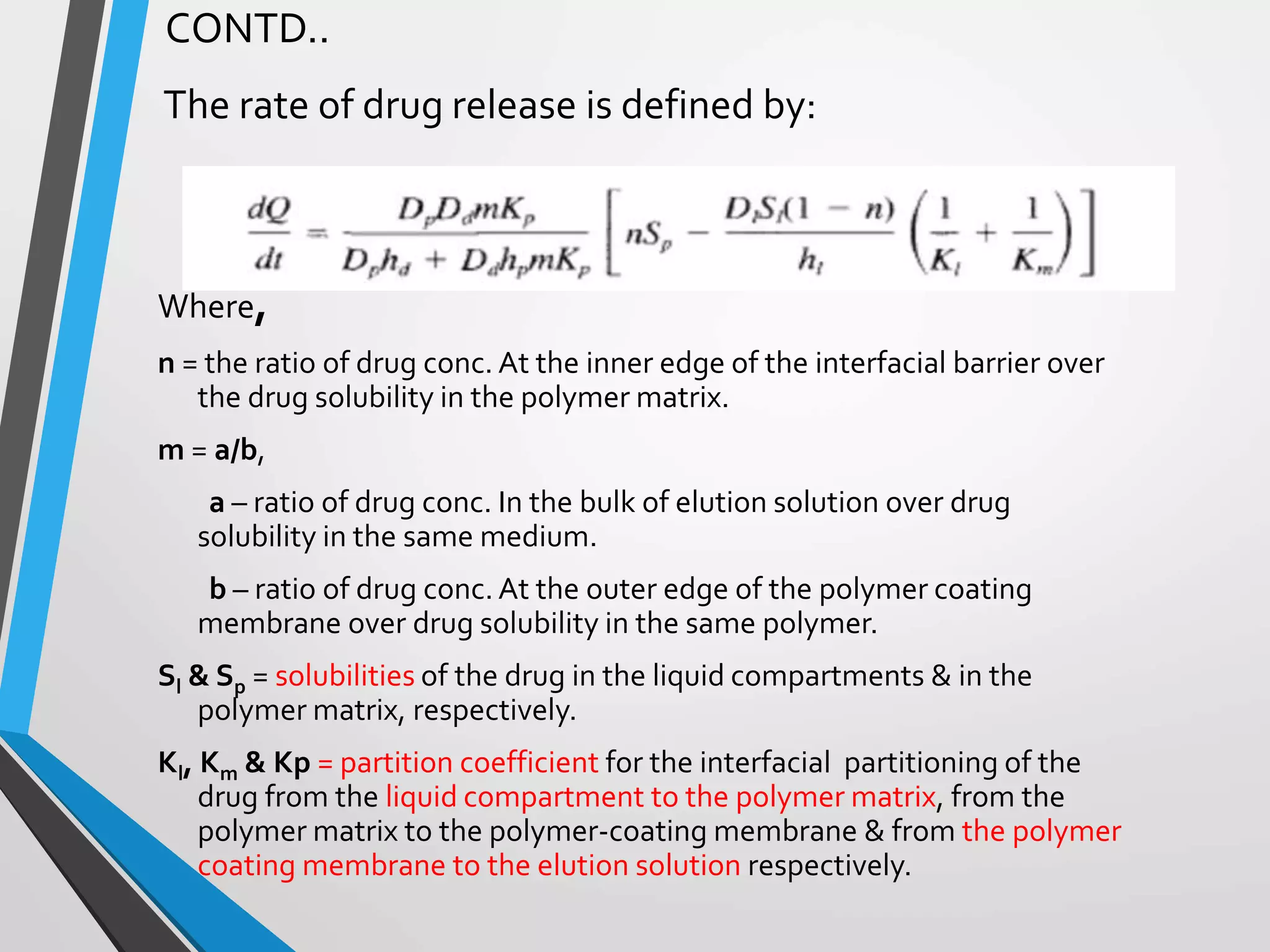
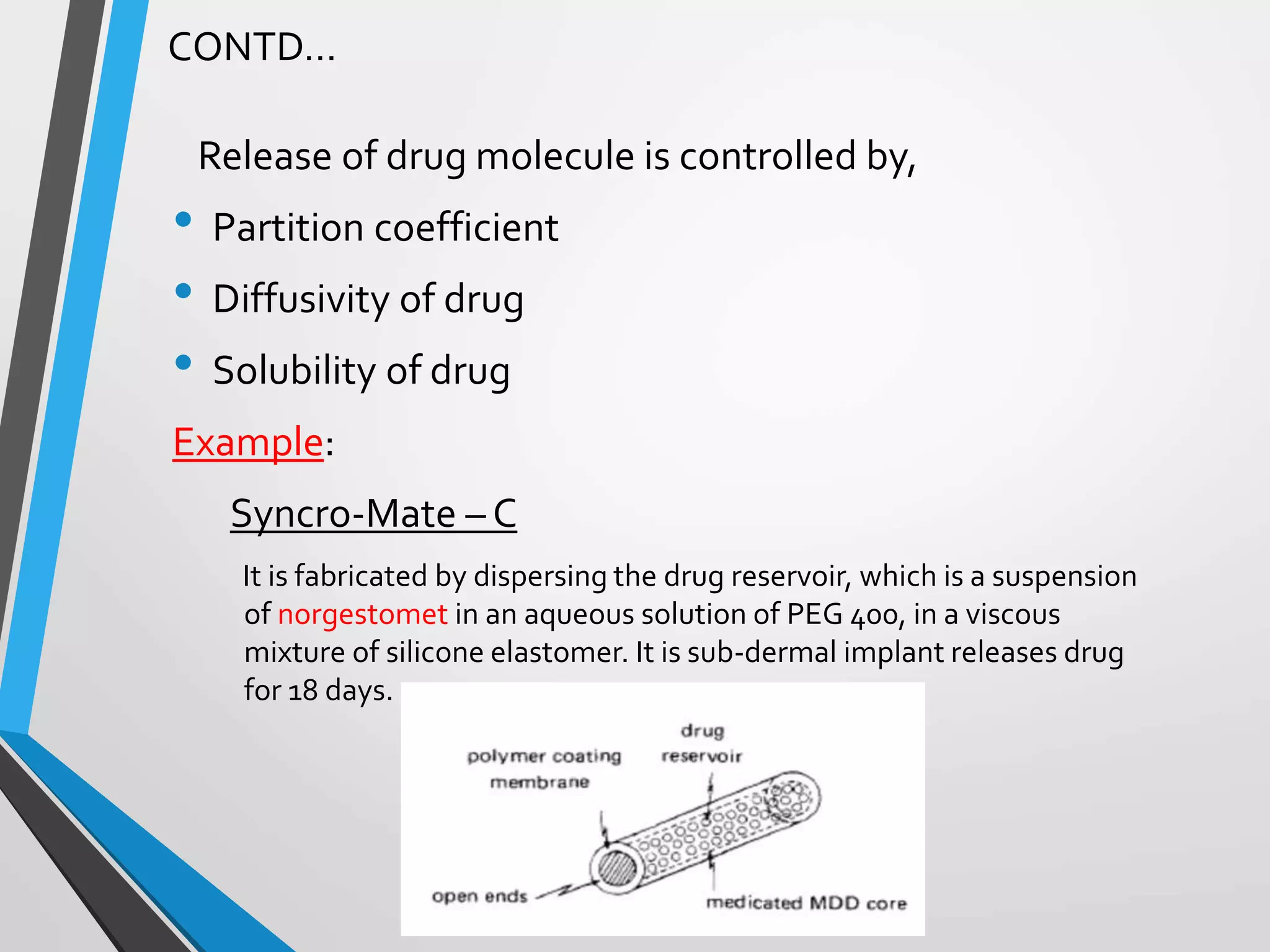
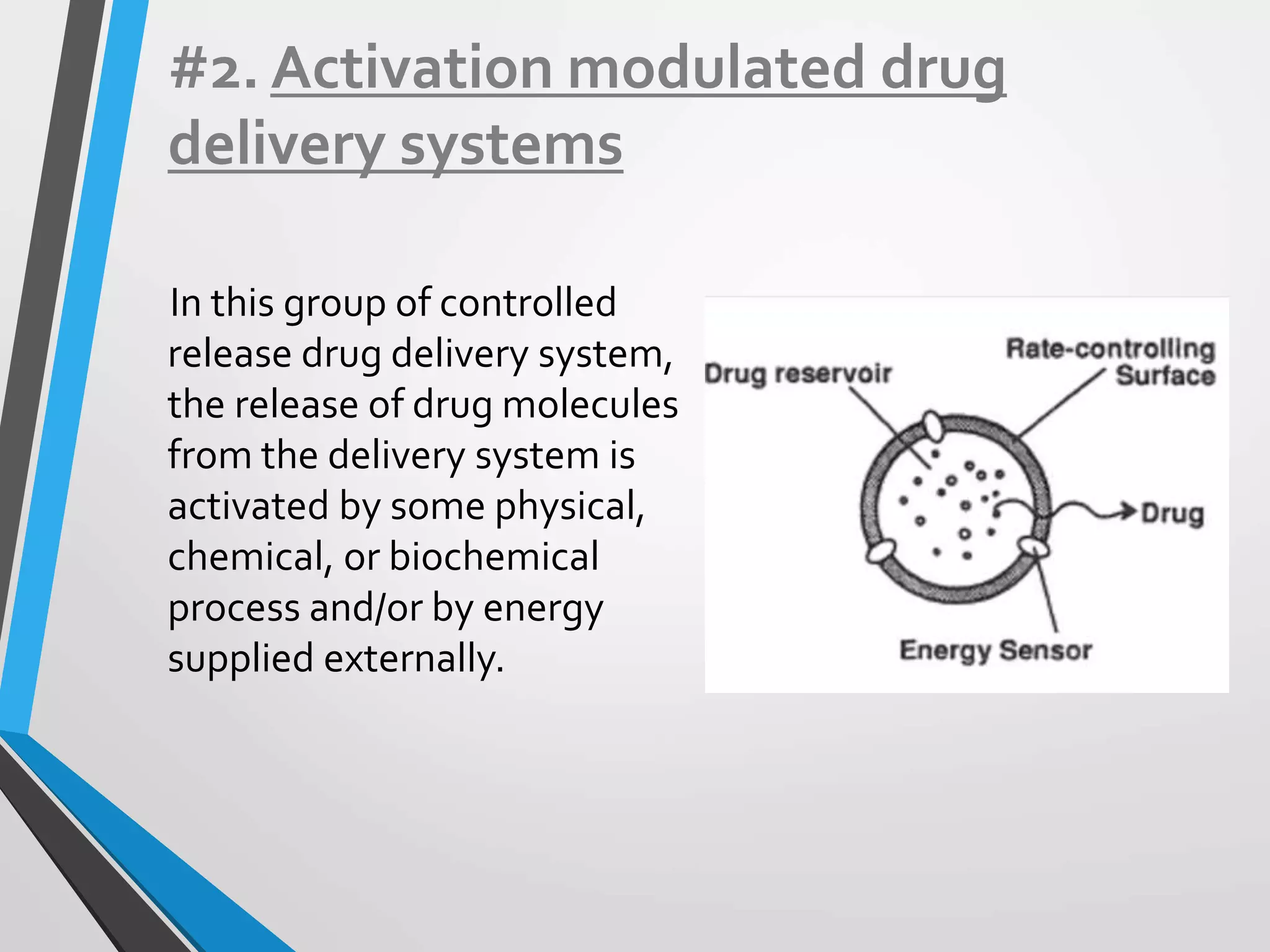
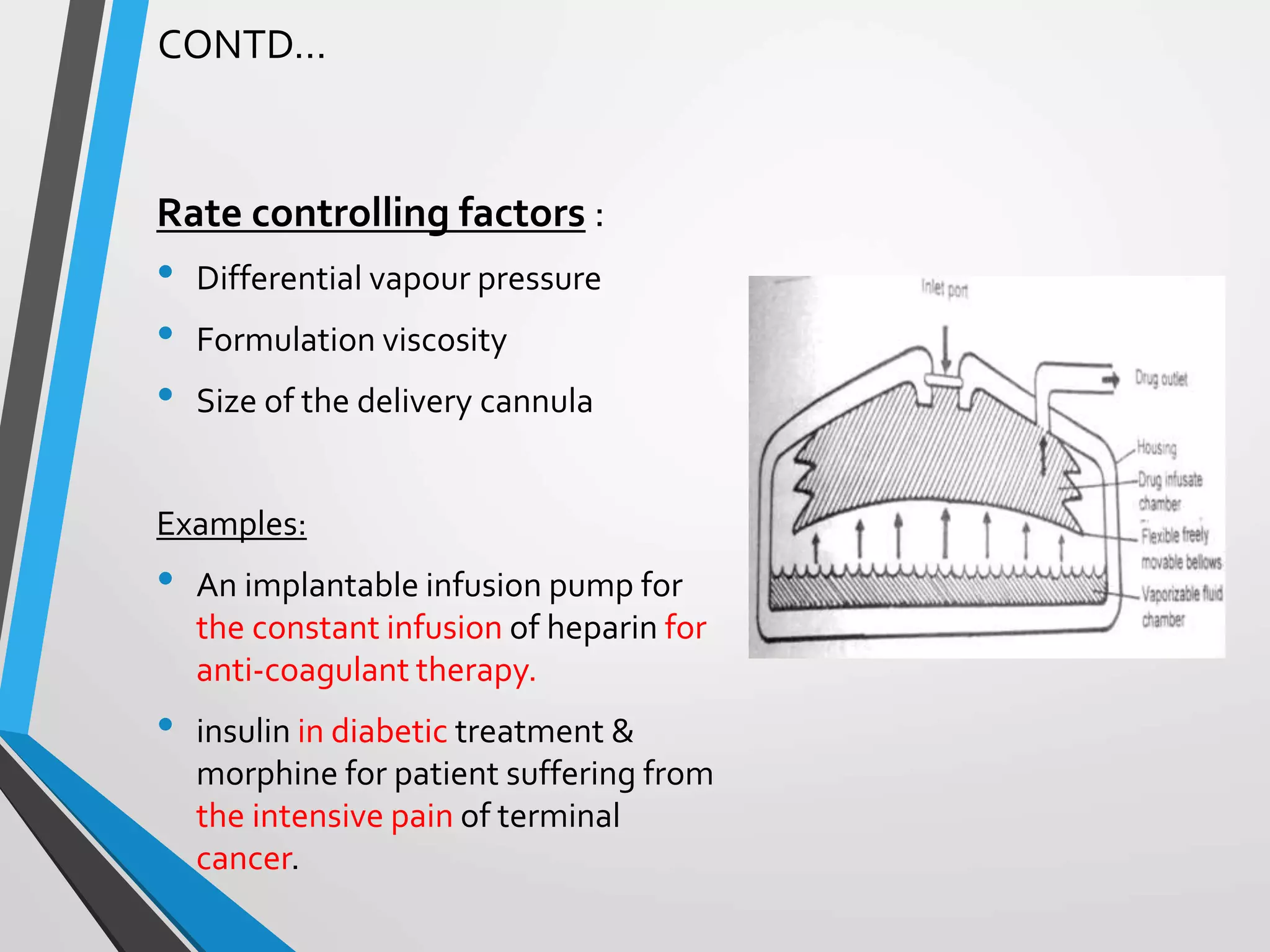
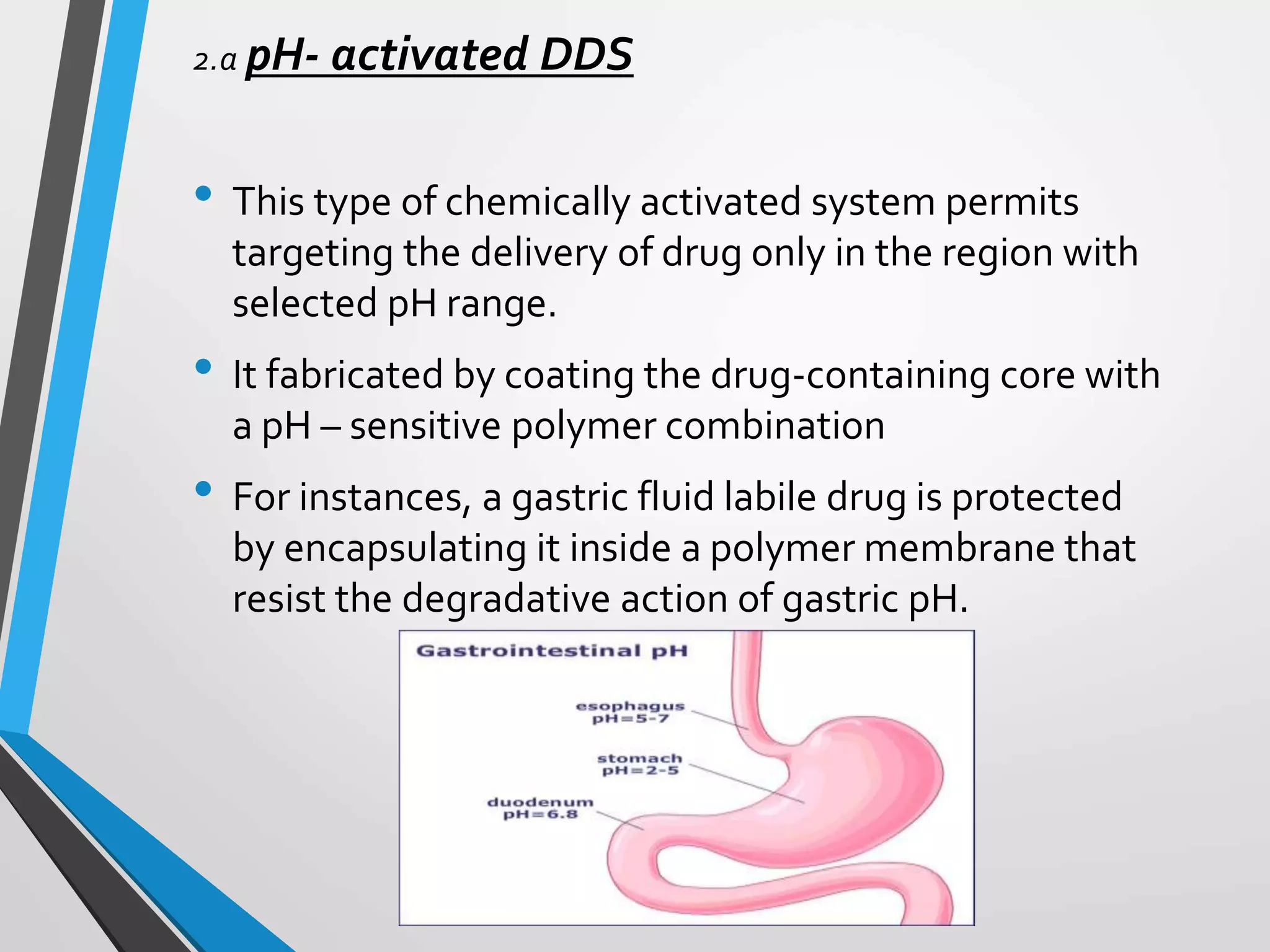
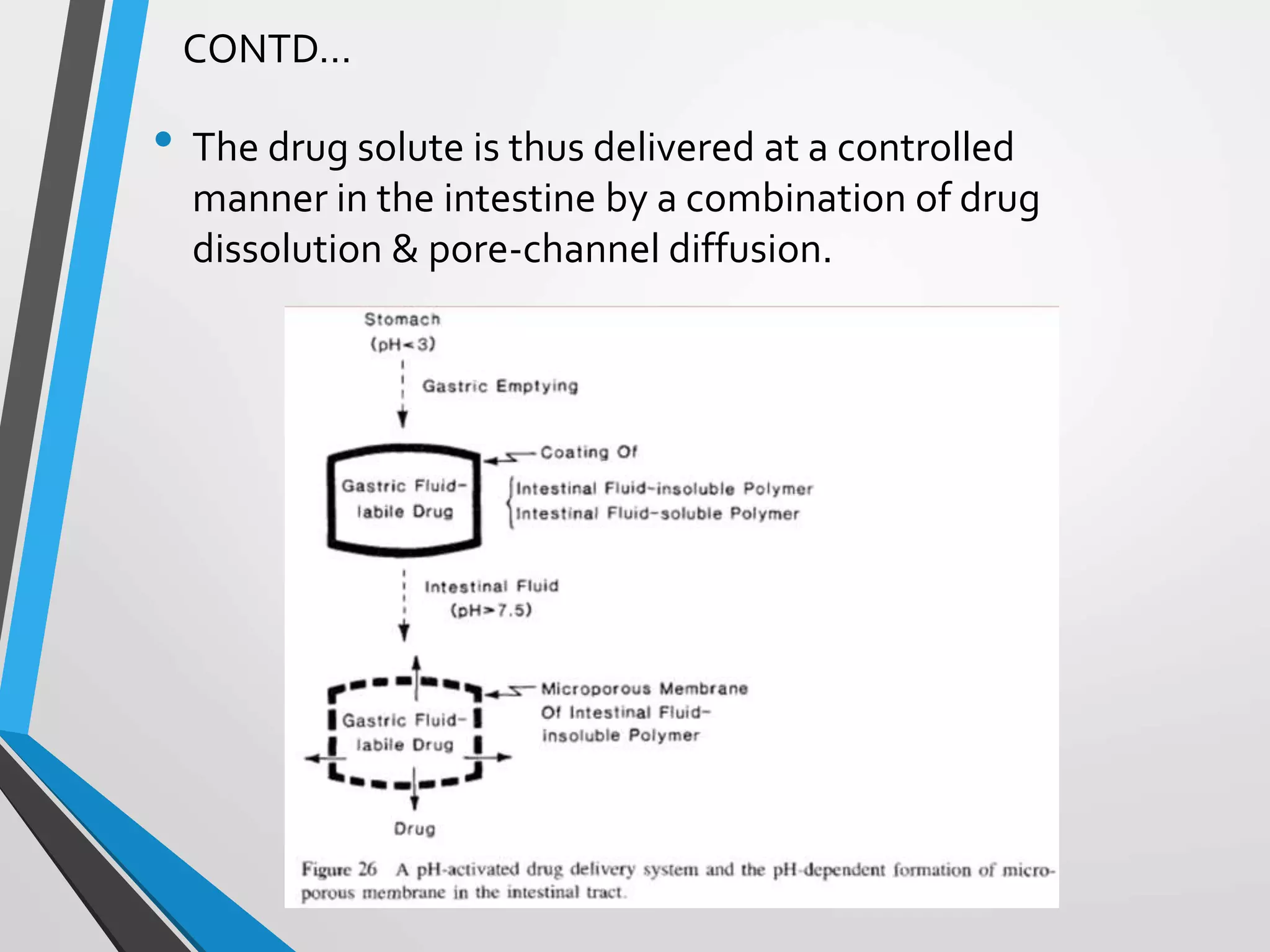
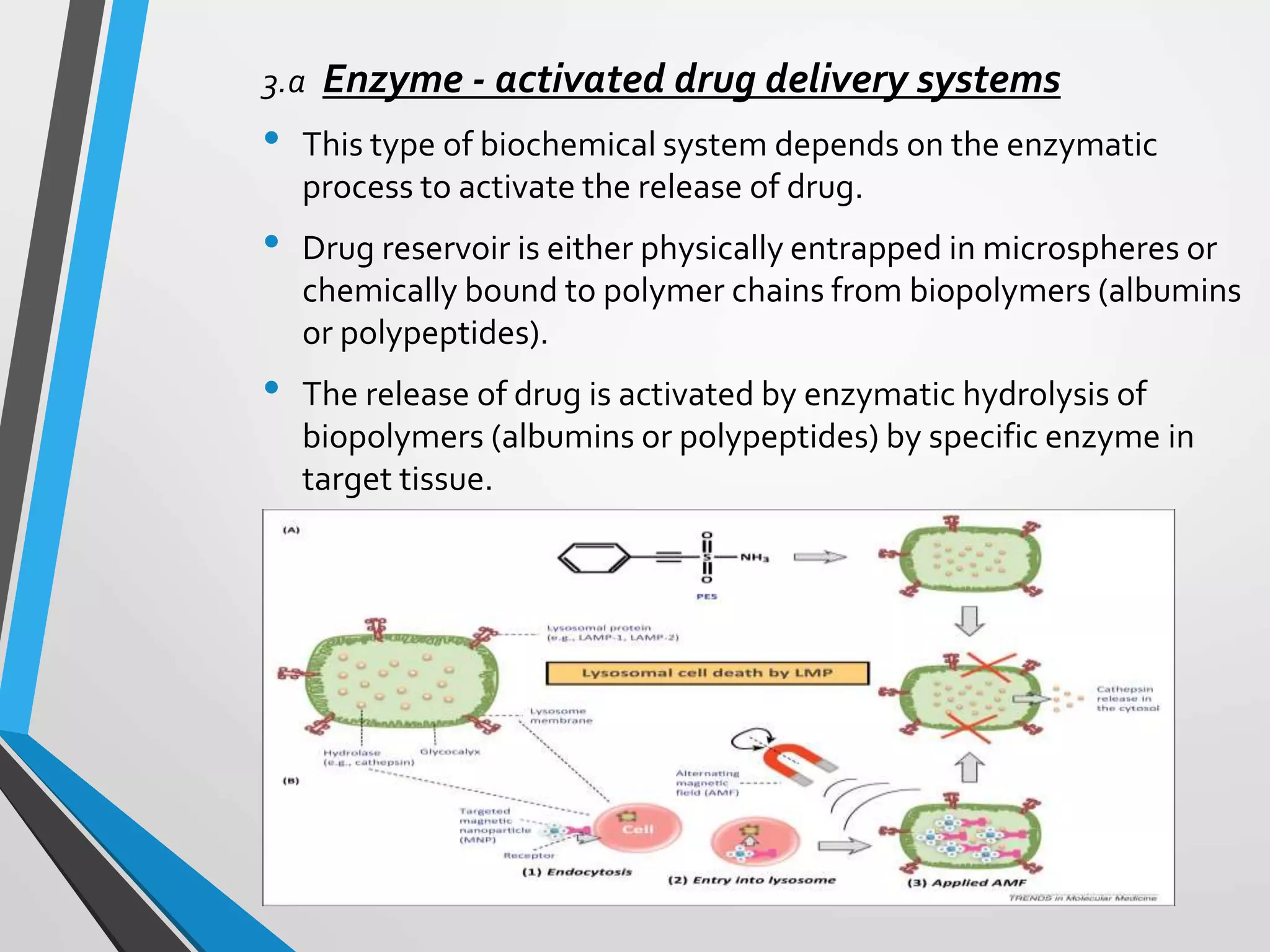
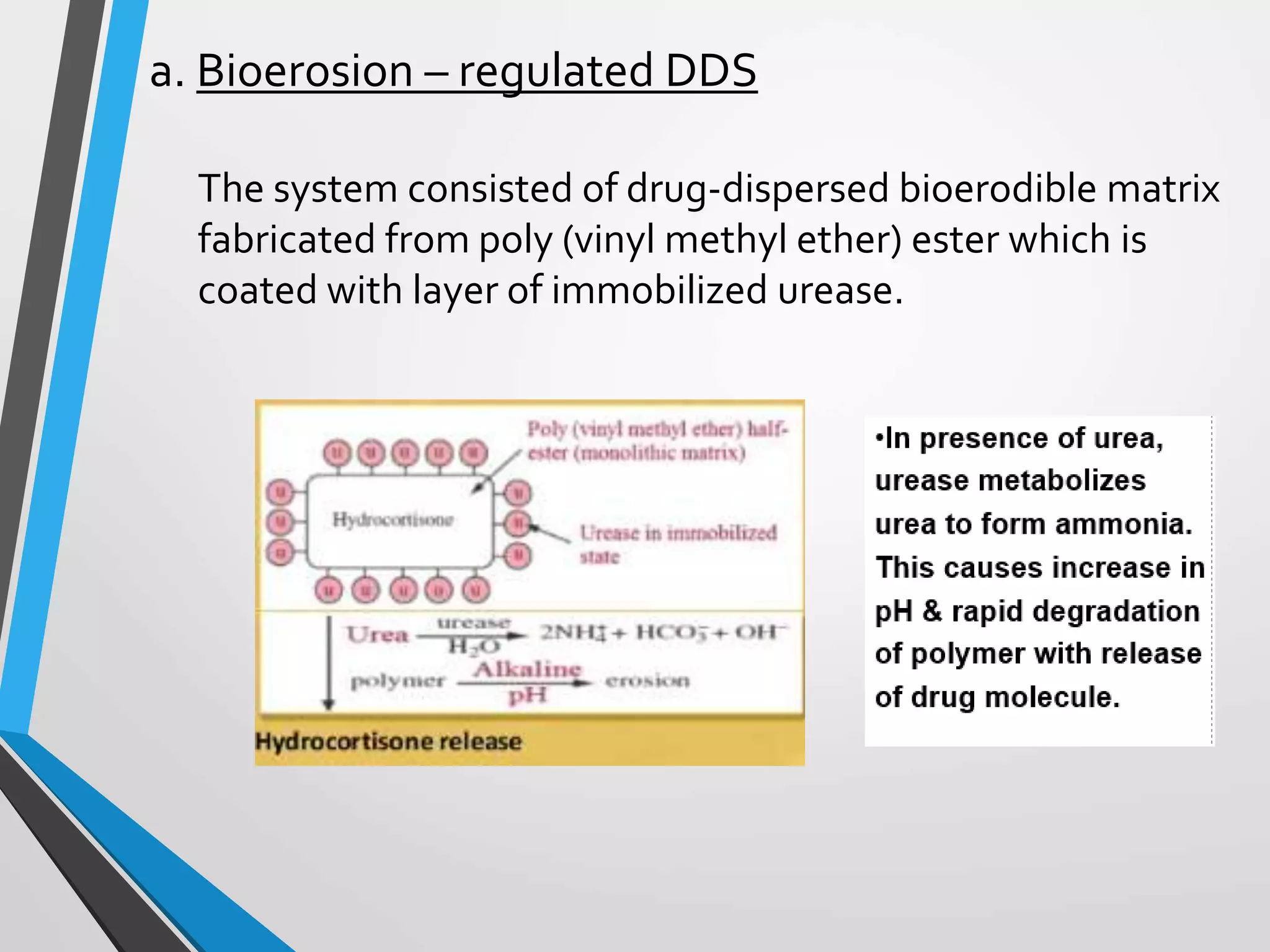
This document discusses different types of rate controlled drug delivery systems. It begins by introducing controlled release drug delivery and distinguishing it from sustained release. It then classifies controlled release systems into three main categories: rate programmed, activation modulated, and feedback regulated systems. Within each category it describes several examples of systems, identifying how drug release is controlled in each case. Key factors that can affect controlled release are also listed. The document aims to provide an overview of controlled drug delivery technologies with classifications and examples.