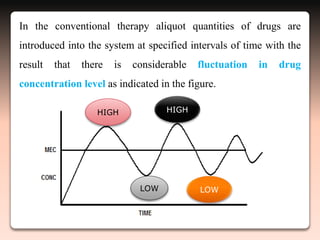
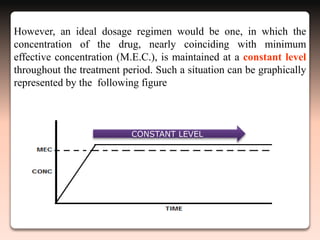
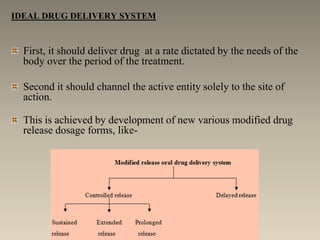
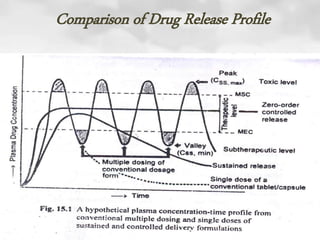
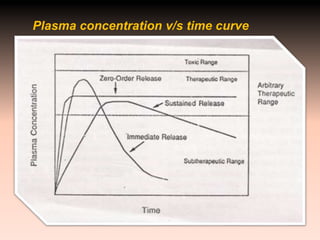
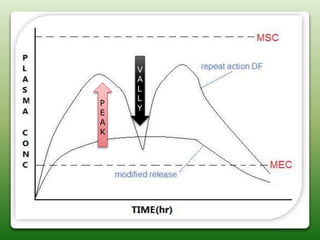
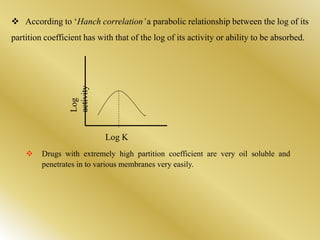
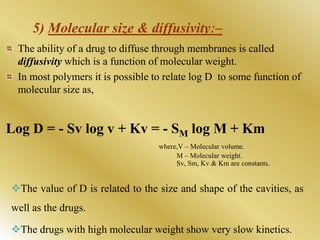
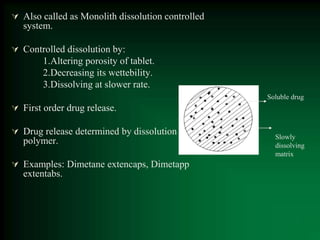
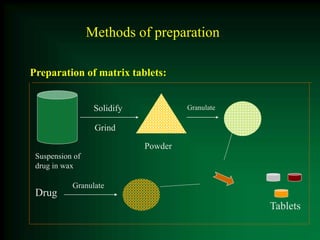
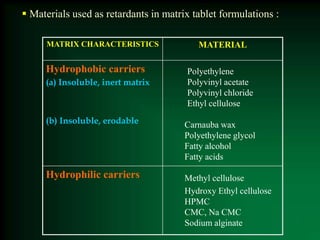
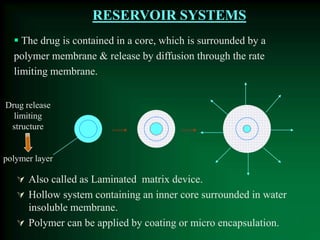
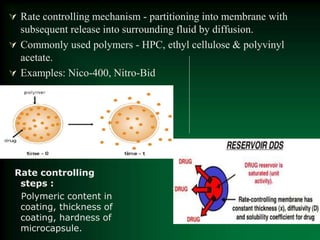
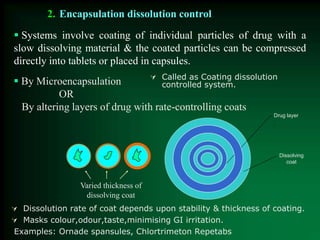
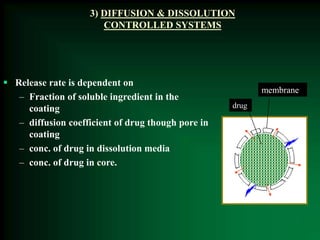
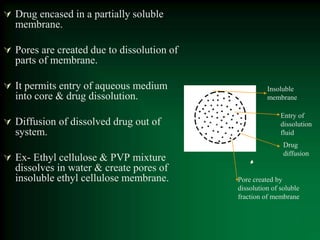
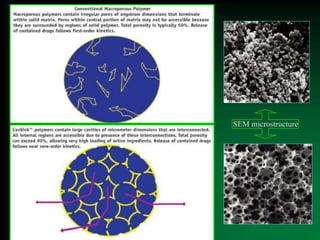
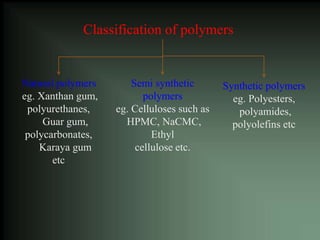
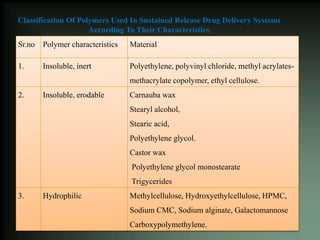
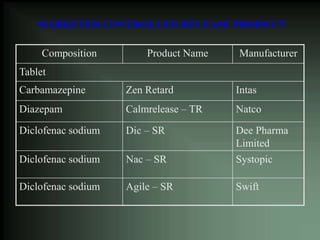
This document discusses oral sustained and controlled release dosage forms. It begins with an introduction and overview of rationality in designing sustained release drug formulations. It defines sustained release as formulations that continuously release medication over an extended period after a single dose to achieve prolonged therapeutic effects. Controlled release aims to deliver drug at a predetermined rate for a specified time period to maintain constant drug levels. The document outlines the differences between controlled and sustained release. It discusses objectives and advantages of sustained release formulations as well as challenges and factors to consider in design.