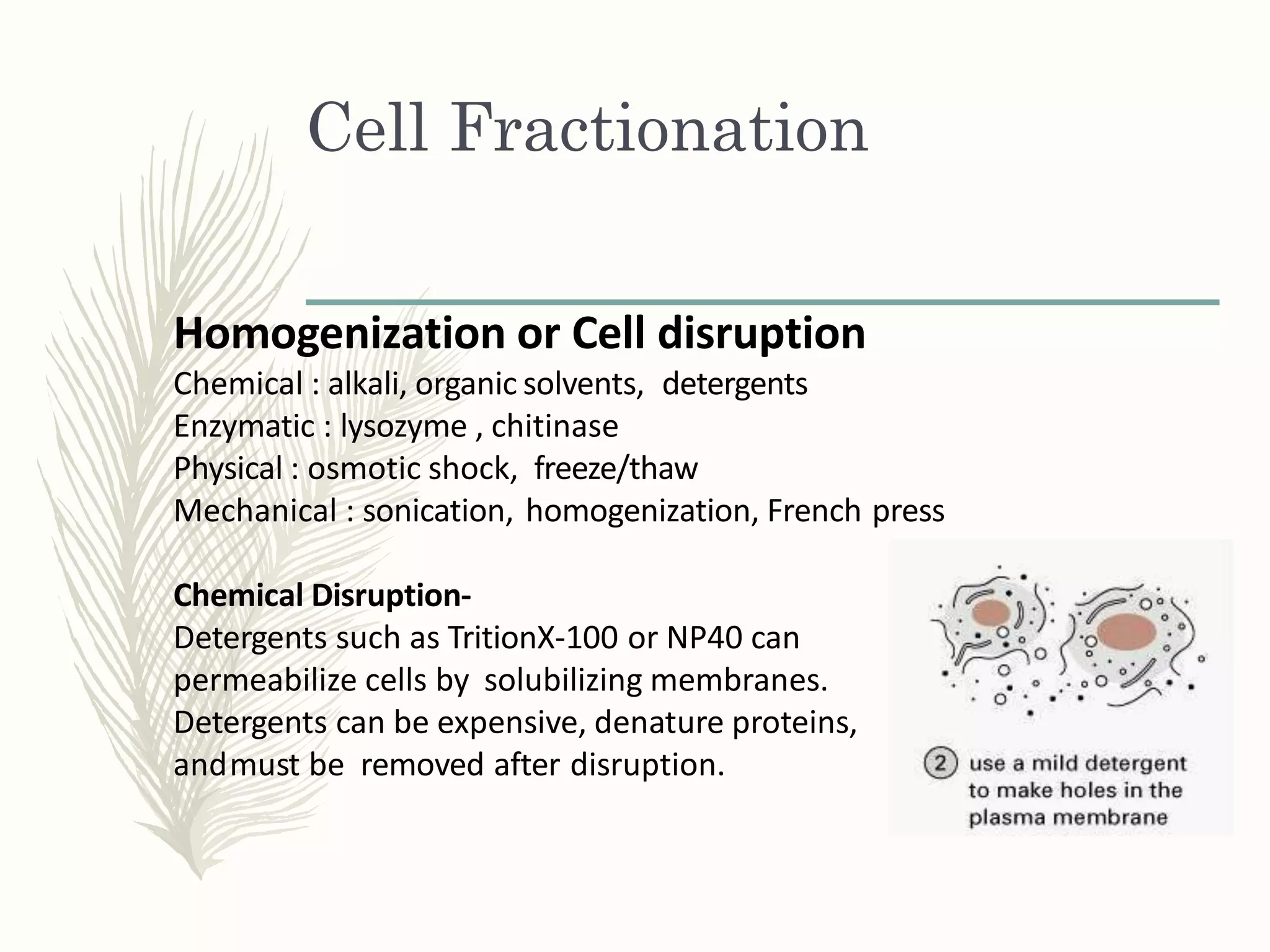
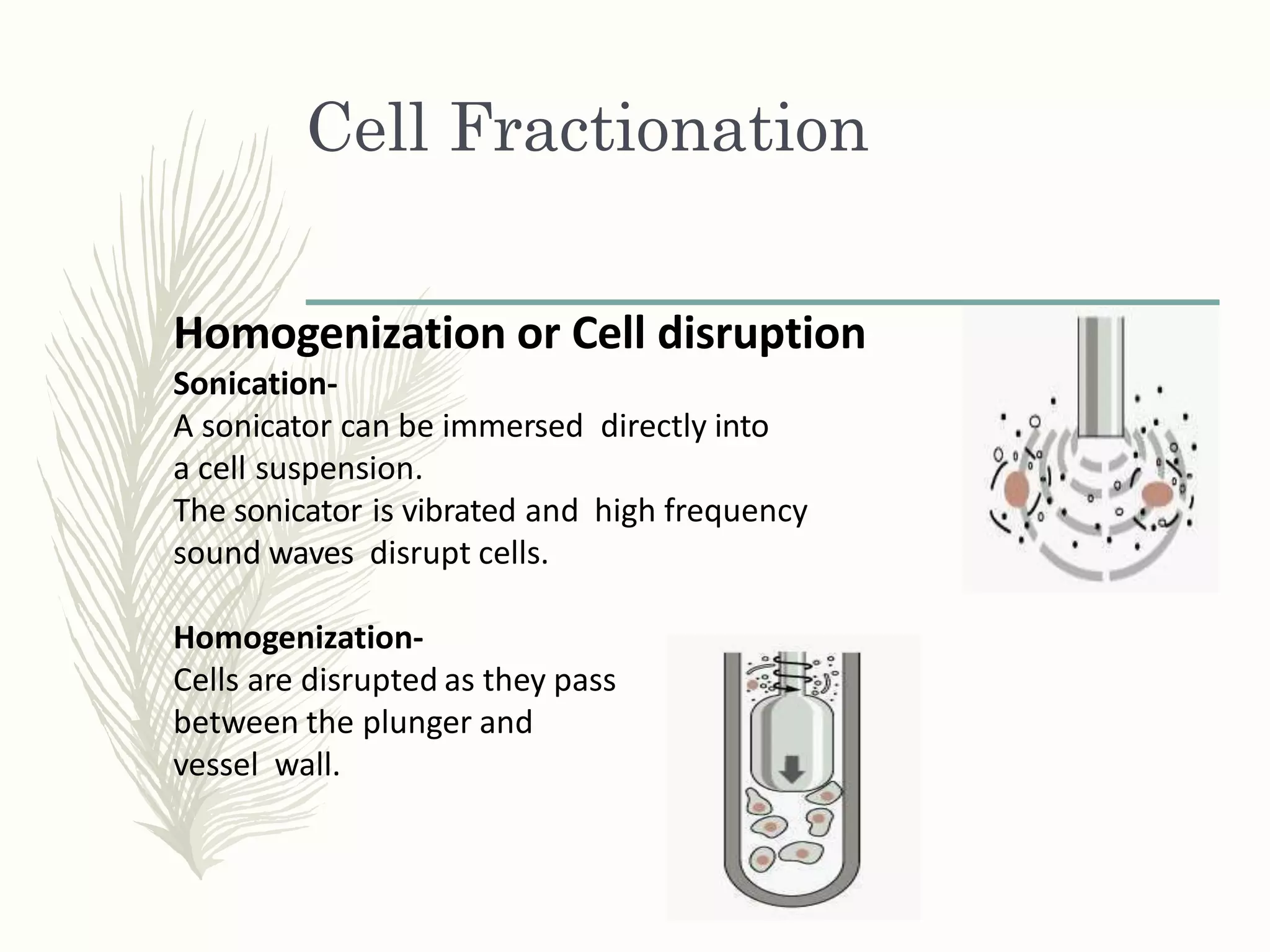
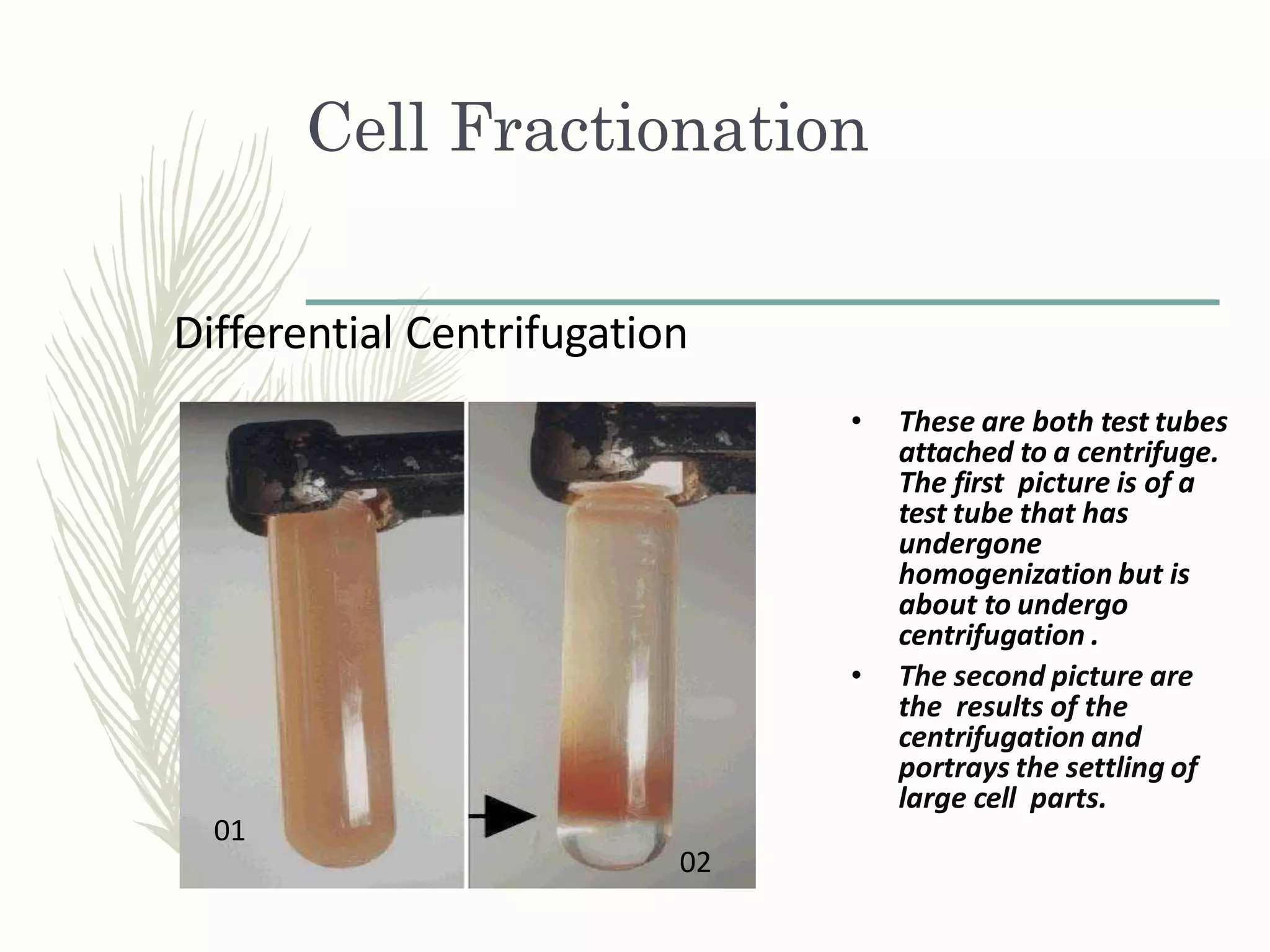
Cell fractionation is a technique used to isolate cell organelles. It involves homogenizing cells and separating the components via differential centrifugation or density gradient centrifugation. This allows organelles to be identified and studied individually using marker enzymes that are specific to each organelle. Cell fractionation has provided insights into the structure and function of organelles and their role in cellular processes.