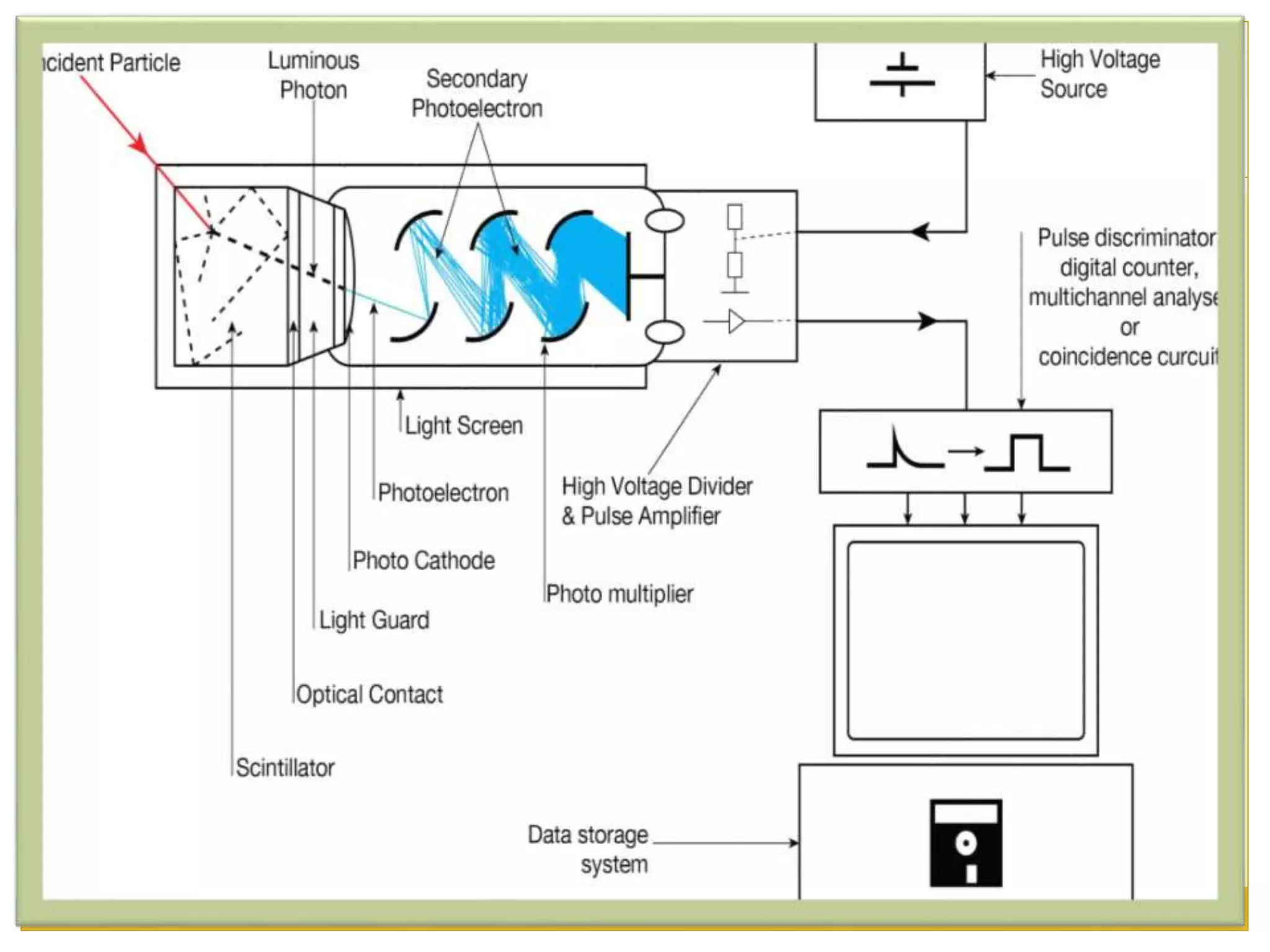
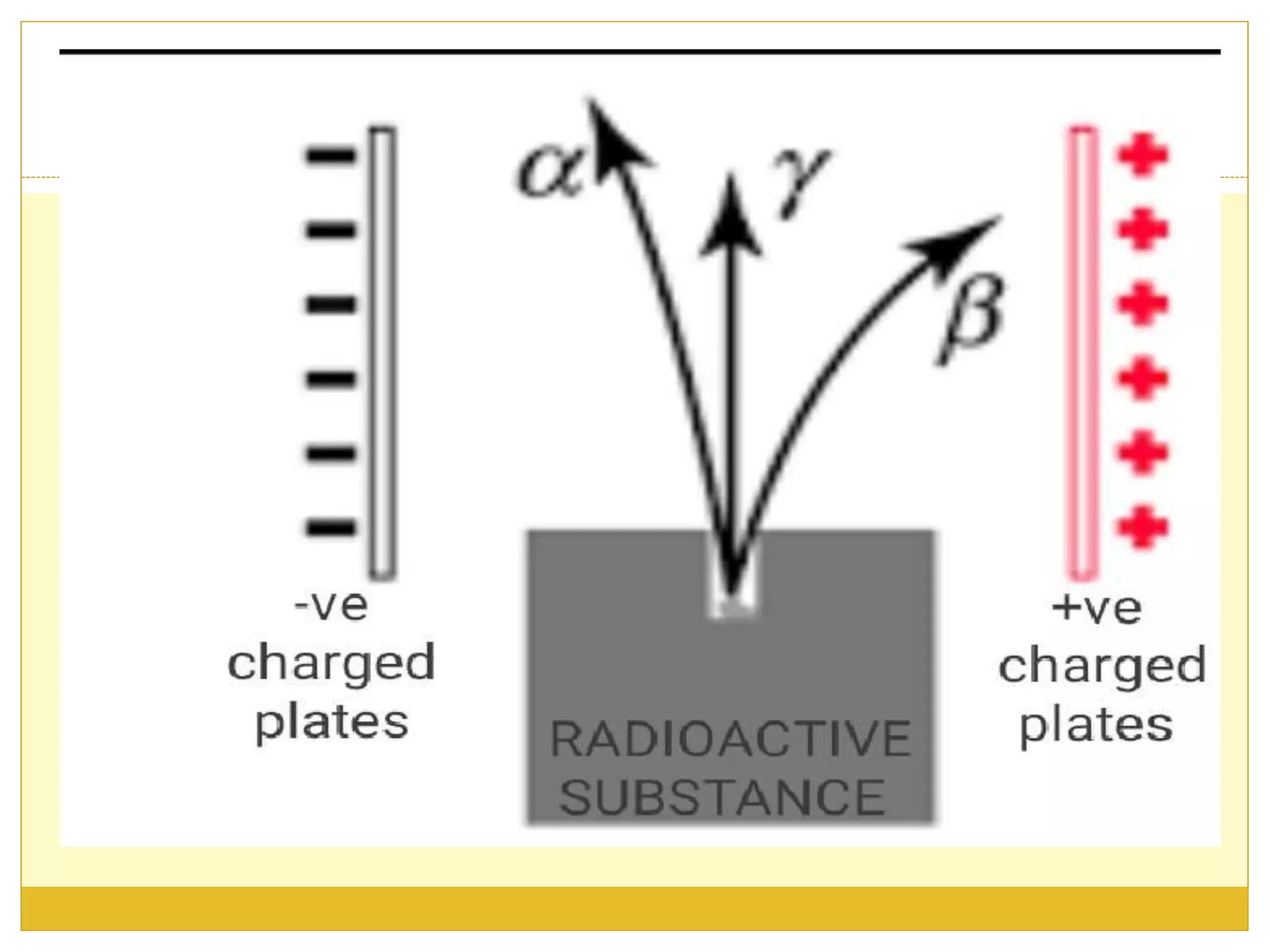
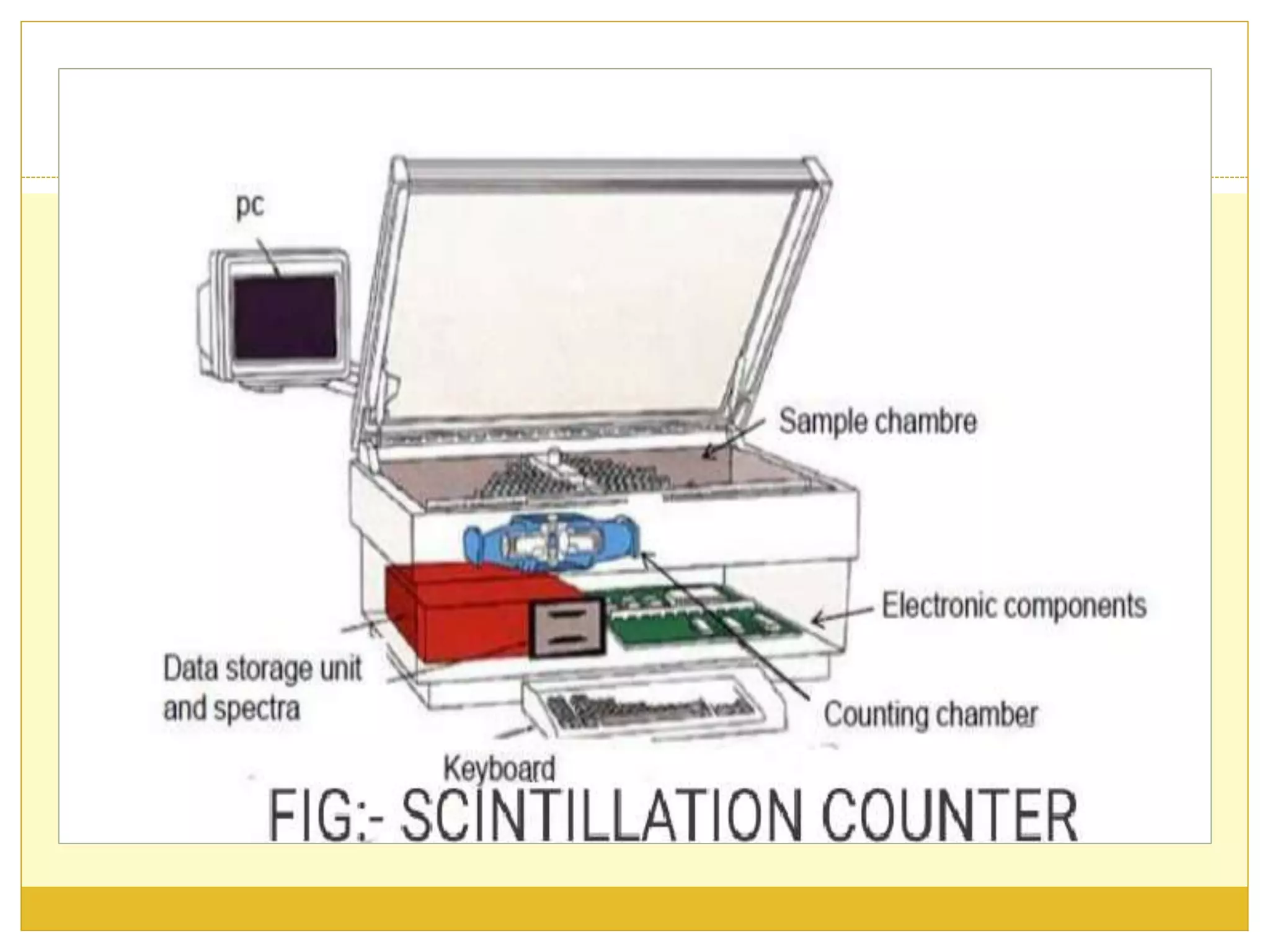
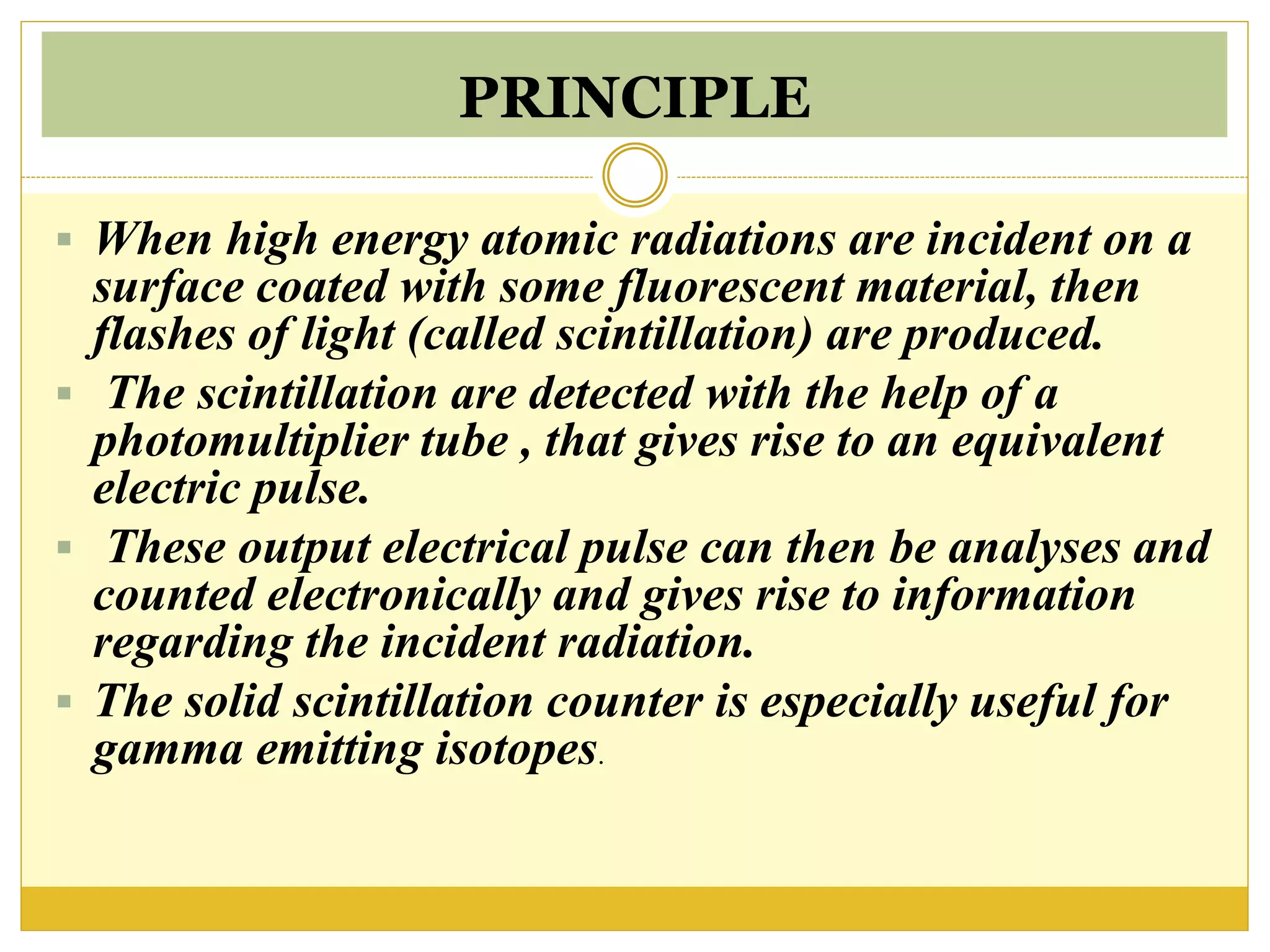
This document discusses solid scintillation counters. It provides background on radioactivity and how scintillation counters can detect and measure different types of radiation. A solid scintillation counter uses a scintillation crystal that produces light flashes when hit by radiation. These light flashes are converted to electrical pulses that can be analyzed electronically. Common scintillation crystals discussed are sodium iodide, zinc sulfide, and anthracene. The document outlines the basic instrumentation, working principle, applications and advantages of solid scintillation counters.





![INSTRUMENTATION
1. RADIATION- The high energy ionizing radiation strike
the crystal.
2. SCINTILLATOR- It consist of a scintillator which
generates photons in response to incident radiation.
3. LIGHT FLASHES- Flash or rays of light produced in a
transparent material by passing a particle.
4. PHOTOMULTIPLIER TUBE [PMT]- It converts the light
to an electrical signal and electronics to process this
signal.
5. PHOTOCATHODE- A photocathode is a surface that
convert light (photons) into electrons by photoelectric
effect.](https://image.slidesharecdn.com/soildscintillationcounterppt-230219152414-b80c372a/75/SOILD-SCINTILLATION-COUNTER-PPT-pptx-15-2048.jpg)